A Review of Exosomes and their Role in The Tumor Microenvironment and Host-Tumor "Macroenvironment"
Kaity H. Tung, Marc S. Ernstoff, Cheryl Allen, Shin La Shu*
Department of Medicine, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
Abstract
Tumor-derived exosomes (TEX) are important intercellular messengers that contribute to tumorigenesis and metastasis through a variety of mechanisms such as immunosuppression and metabolic reprogramming that generate a pre-metastatic niche favorable to tumor progression. Our lab has contributed further to the understanding of the miRNA payloads in TEX by demonstrating that human melanoma-derived exosome (HMEX) associated miRNAs contribute to the metabolic reprogramming of normal stroma. This mini-review highlights the role of TEX in the tumor microenvironment (TME) and the hypothesis that exosomes may also generate a host-tumor “macroenvironment” beyond the TME through their miRNA and protein payloads, so to speak “fertilizing the soil for cancer seeding.”
Introduction
Exosomes are cell-derived nano-meter sized (40-100 nm) particles that have been established to play an important role in cell-to-cell communication. Most, if not all, cells produce exosomes. Exosomes are part of a broader network by which cells communicate between each other by shuttling DNA, RNA, proteins and membrane bound factors. Tumor-derived exosomes (TEX) have profound impact on the immediate tumor microenvironment (TME). Moreover, by virtue of their distribution through the blood and lymph, TEX can play a role at distant tissue sites to create a pre-metastatic niche conducive to metastasis, which we refer to as the tumor “macroenvironment” (TMaE). TEX deliver tolerogenic signals to immune cells, inhibiting immune cell proliferation, inducing apoptosis of activated CD8+ T lymphocytes, interfering with monocyte differentiation and promoting the expansion of regulatory T cells thus inducing immune suppression through a paracrine effect1. Interestingly, TEX display certain ligands, such as programmed death-ligand 1 (PD-L1), to produce an endocrine signaling effect to generate a favorable pre-metastatic TMaE, extending a distance away from the primary tumor. Chen et al. showed that PD-L1 expression is enhanced when melanoma cells are exposed to interferon-ϒ (IFNϒ), which leads to enhanced expression of PD-L1 on the surface of circulating human-derived melanoma exosomes (HMEX)2. Our lab has recently demonstrated that HMEX can also metabolically reprogram normal stroma through their microRNA (miRNA) payload causing a switch from oxidative phosphorylation (OXPHOS) to glycolysis and an increase in extracellular acidification3. Here we review the role of exosomes on the TME and extend these observations by proposing that exosomes can prepare host organs by creating a host-tumor “macroenvironment” that generates a fertile environment for metastasis (Figure 1).
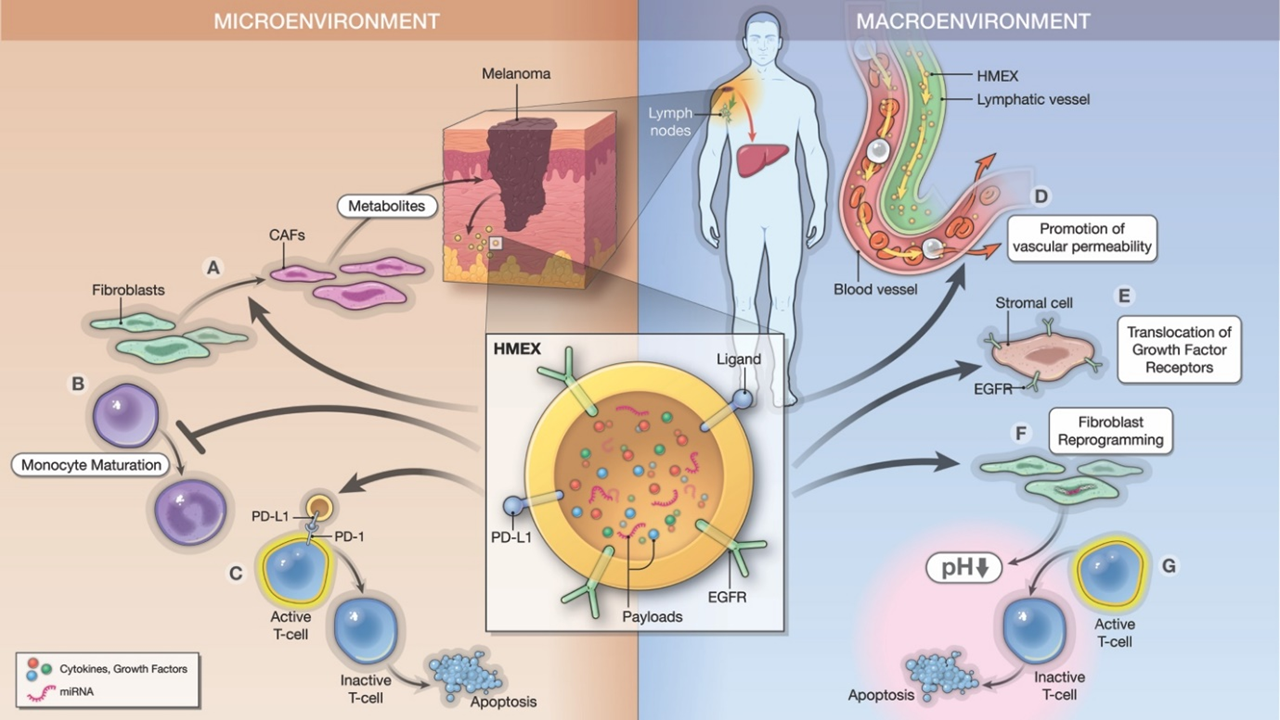
Figure 1: Roles of cancer-derived exosomes in generation of tumor “microenvironment” and “macroenvironment.”
Cancer-derived exosomes participate in the generation of a tumor microenvironment (TME) through paracrine signaling and in an endocrine pathway that can influence other parts of the body, referred to as a tumor macroevironment (TMaE). Here, we use a human melanoma model to demonstrate the mechanisms in which human melanoma-derived exosomes (HMEX) use in developing a pre-metastatic niche. In the immediate TME, HMEX are released from the primary tumor to convey immunosuppression. (A) HMEX contributes to the transition of normal fibroblasts to cancer-associated fibroblasts (CAFs). (B) They also suppress monocyte maturation and induce a monocytic myeloid-suppressor cell phenotype. (C) Moreover, they display ligands (ex. programmed death-ligand 1 (PD-L1)), that inactivate T-cells through direct binding. (D) We also hypothesize that HMEX promote a pre-metastatic niche at a distant site (ex. liver) through bloodstream and lymphatic drainage to promote vascular permeability, immunosuppression and metastasis. (E-G) There, HMEX act on normal stroma by translocation of growth factor receptors (ex. EGFR) and metabolic reprogramming using microRNA (miRNA) payloads (ex. miR-155, miR-210), exhibiting a reverse Warburg effect and promoting extracellular acidification that contribute to the anergy of CD8+ T cells. In conclusion, HMEX not only create a favorable immediate environment but a macroenvironment to facilitate the metastatic process.
Background
The TME is composed of two components: cellular and acellular. The cellular component consists of fibroblasts, endothelial cells, pericytes and immune cells. The acellular component consists of extracellular matrix, soluble factors and extracellular vesicles4. Exosomes are a type of extracellular vesicle (EV), and they play a major role in cell-cell communication as key messengers that regulate physiological processes. EVs are membrane vesicles that vary in size from exosomes (40-100 nm), microvesicles (50-1000 nm) to apoptotic bodies (800-5000 nm), and are capable of shuttling proteins, nucleic acids, metabolites and lipids between cells. Exosomes, in particular, have been widely studied in the recent years as they were discovered to be a mechanism by which tumor cells enhance progression and metastasis. They are found in the supernatant of cultured cells as well as body fluids. Thus, they have the ability to act locally as well as distally, influencing and modifying the stroma in the TME.
Stephen Paget first introduced the hypothesis of seed and soil in the context of cancer metastasis in 1889, referring to the observation that certain organ sites appear to be as fertile soil for seeding by cancer cells. This highlights the non-random pattern of metastasis5. We extend this observation by suggesting that exosomes can act in a macroenvironment fashion and function as a “fertilizer” for that soil, creating a pre-metastatic niche at a site distant from the primary tumor to enable the seeding.
TEX can mediate immune suppression through a paracrine effect
Tumor cells may create a favorable pre-metastatic niche by driving immune suppression6. We have demonstrated that one pathway in creating a pre-metastatic niche is through TEX metabolically reprogramming normal fibroblasts and generating an acidic microenvironment detrimental to immune function. Other pathways include direct engagement of TEX with receptors on immune cells. Muller et al. showed that TEX are not readily internalized by T cells, but instead express ligands that engage receptors on T cells, including the T cell receptor (TCR) and IL-2 receptor (IL-2R) on T cells to cause alterations in T cell protein expression and transcriptome7. Taylor and colleagues demonstrated that TEX drive the apoptosis of T lymphocytes and induce the loss of TCR-associated signal transducing ζ-chain expression causing deficient T cell-mediated immune responsiveness in cancer patients8. TEX inhibit the IL-2 proliferative response in CD8+ T cells and favor regulatory T cell responses9, 10. TEX can also suppress monocyte maturation and generate a monocytic myeloid-derived suppressor cell phenotype (Mo-MDSC) that is favorable to tumor escape from immune recognition11. This set of evidence magnifies the modification on the immediate TME by TEX through paracrine signaling to promote immune escape, tumorigenesis, and thus “fertilizing” the sites for metastasis.
TEX promote the establishment of a tumor “macroenvironment” through an endocrine effect
Several studies have shown that TEX have an endocrine signaling effect, establishing a host-tumor “macroenvironment” away from the primary tumor site through several mechanisms. TEX membrane expression of PD-L1 is similar to tumor cell expression of PD-L1. TEX PD-L1 can suppress T cell activation and enhance tumor specific immune suppression2. Chen et al. demonstrated a correlation between a higher level of circulating exosomal PD-L1 and poorer clinical outcomes after immune checkpoint inhibitor therapy. Exosomes are also involved directly in manipulating stromal cells. For instance, epidermal growth factor receptor (EGFR) can be translocated from gastric cancer exosomes to liver stromal cells to activate hepatocyte growth factor12. Furthermore, Peinado et al. demonstrated that HMEX have the ability to induce vascular leakiness at pre-metastatic sites, and importantly have the ability to reprogram bone marrow progenitor cells to gain a pro-vasculogenic phenotype through the MET receptor13. Recently, our lab has shown another mechanism by which cancer exosomes can modify the pre-metastatic niche. Shu et al. showed that HMEX are capable of reprogramming the metabolism of normal stromal fibroblasts by skewing the dominant metabolic process from OXPHOS to aerobic glycolysis, encompassing the Warburg effect and inducing extracellular acidification that has been shown to contribute to a pre-metastatic niche and a state of anergy of CD8+ T lymphocytes3,14, 15. This phenomenon also contributes to the two compartmental microenvironment process referred to as the reverse Warburg effect16. We emphasize that HMEX can promote extracellular acidification to favor pre-metastatic soil locally as well as distally through distribution via the vascular and lymphatic systems to alter normal stromal cells they contact.
TEX establish a pre-metastatic tumor “macroenvironment” through their miRNA payload
TEX mediate immunosuppression and cellular reprogramming genetically and metabolically through their microRNA (miRNA) payload. miRNAs are small non-coding single-stranded RNAs that range from 19-24 nucleotides long that can negatively regulate post-translational mRNAs17. Our lab demonstrated the capability of HMEX to metabolically reprogram normal stroma through two exosomal miRNAs, miR-155 and miR-210 by using a tethered cationic lipoplex nanoparticle (tCLN) biochip described by Wu et al.18. tCLN biochip is a novel technology that utilizes a cationic lipoplex to capture cell-secreted exosomes by electrostatic interactions. The resulting lipoplex/exosome complex then allows molecular beacons within the lipoplex to bind to target exosomal RNA, emitting fluorescent signals for detection. As noted, we also demonstrated that HMEX are involved with two compartment metabolic coupling between cancer and stromal cells known as the reverse Warburg effect. As noted HMEX induce aerobic glycolysis in the stromal cells will in turn generate high energy fuel such as pyruvate, lactate, ketones bodies and fatty acids to sustain the metabolic needs of the cancer3, 19.
Ye et al. identified a cluster of nasopharyngeal carcinoma derived exosome associated miRNAs (hsa-miR-24-3p, hsa-miR-891a, hsa-miR-106a-5p, hsa-miR-20a-5p and hsa-miR-1908) that downregulate MAPK1 and JAK/STAT signaling pathways in T cells, contributing to immune suppression20. Ding et al. revealed that pancreatic cancer (PC)-derived exosomes carry miR-212-3p, which is responsible for the inhibition of regulatory factor X-associated proteins (RFXAP), an important transcription factor for MHC-II that can induce immune tolerance in the TME21. Taken together, these observations strongly support the hypothesis that TEX have the capability to reprogram stromal and immune cells through their miRNA payloads establishing a nonrandom metastatic niche for cancer in the TME and beyond.
TEX can induce the transition of normal stromal cells to cancer-associated fibroblasts (CAFs)
CAFs are morphologically similar to myofibroblasts and provide another TME pathway of ongoing support for the cancer niche22, 23. Our lab has confirmed the work of others that TEX can also play a pivotal role in transforming normal stromal cells to CAFs through TGF-β24. CAFs can be characterized by expression of α-smooth muscle actin (α-SMA) and fibroblast activating protein (FAP). Wei and colleagues demonstrated malignant ascites-derived exosomes promote proliferation and induce the transition of normal mesothelial cells into CAFs25, and Ning and co-authors showed that gastric cancer (GC)-derived exosomes induce pericytes into CAFs following internalization26. In addition, Paggetti et al. revealed that chronic lymphocyte leukemia cell-derived exosomes induce CAFs from bone marrow-mesenchymal stem cells (BM-MSCs) and endothelial cells (ECs)27. These studies further demonstrated the role of exosomes in the process of setting up a favorable soil, forming a pre-cancerous “macroenvironment” for the seeding of cancer cells.
Conclusion
TEX are important messengers that enhance tumorigenesis and metastasis. They achieve this through a variety of mechanisms including immunosuppression, and molecular and metabolic reprogramming that create a pre-metastatic niche to facilitate the process of tumor progression. Studies have shown that TEX not only influence the immediate TME, but they also travel to distant tissue sites to establish a host-tumor “macroenvironment” through an endocrine phenomenon. Our lab has contributed further to the understanding of the miRNA payloads in TEX that HMEX miR-155 and miR-210 contribute to the metabolic reprogramming of normal stroma.
We posit that exosome distribution through the vascular and lymphatic systems can allow TEX to enhance the TMaE beyond the immediate TME, extending the seed and soil hypothesis by concluding that the soil may be prepared prior to seeding via cancer exosomes and their miRNA and protein payloads.
Acknowledgements
This work was supported by NIH training grant T35 AI089693, National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Comprehensive Cancer Center’s Flow and Image Cytometry, Genomics, Biostatistics (etc) Shared Resources and was internally sponsored by Roswell Park Comprehensive Cancer Center. We are remarkably grateful to Yana Hammond of ATLAS Studios (https://www.roswellpark.org/shared-resources/atlas-studios) for assistance with the Illustration.
References
- Whiteside TL. Exosomes and tumor-mediated immune suppression. The Journal of Clinical Investigation. 2016; 126(4): 1216–1223.
- Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018; 560(7718): 382-386.
- La Shu S, Yang Y, Allen CL, et al. Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Scientific Reports. 2018; 8: 12905.
- Hui L, Chen Y. Tumor microenvironment: Sanctuary of the devil. Cancer Letters. 2015; 368(1): 7-13.
- Langley RR, Fidler IJ. The seed and soil hypothesis revisited - the role of tumor-stroma interactions in metastasis to different organs. International Journal of Cancer. Journal International Du Cancer. 2011; 128(11): 2527–2535.
- Sceneay J, Parker BS, Smyth MJ, et al. Hypoxia-driven immunosuppression contributes to the pre-metastatic niche. Oncoimmunology. 2013; 2(1): e22355.
- Muller L, Mitsuhashi M, Simms P, et al. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Scientific Reports. 2016; 6: 20254.
- Taylor DD, Gercel-Taylor C, Lyons KS, et al. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-con- taining membrane vesicles shed from ovarian tumors. Clin Cancer Res. 2003; 9(14): 5113–5119.
- Clayton A, Mitchell JP, Court J, et al. Human Tumor-Derived Exosomes Selectively Impair Lymphocyte Responses to Interleukin-2. Cancer Research. 2007; 67(15): 7458-7466.
- Wieckowski EU, Visus C, Szajnik M, et al. Tumor-Derived Microvesicles Promote Regulatory T Cell Expansion and Induce Apoptosis in Tumor-Reactive Activated CD8+ T Lymphocytes. Journal of Immunology (Baltimore, Md.: 1950). 2009; 183(6): 3720–3730.
- Domenis R, Cesselli D, Toffoletto B, et al. Systemic T Cells Immunosuppression of Glioma Stem Cell-Derived Exosomes Is Mediated by Monocytic Myeloid-Derived Suppressor Cells. PLoS ONE. 2017; 12(1): e0169932.
- Zhang H, Deng T, Liu R, et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nature Communications. 2017; 8: 15016.
- Peinado H, Ale?kovi? M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature Medicine. 2012; 18(6): 883–891.
- Asgharzadeh MR, Barar J, Pourseif MM, et al. Molecular machineries of pH dysregulation in tumor microenvironment: potential targets for cancer therapy. BioImpacts?: BI. 2017; 7(2): 115–133.
- Bellone M, Calcinotto A, Filipazzi P, et al. The acidity of the tumor microenvironment is a mechanism of immune escape that can be overcome by proton pump inhibitors. OncoImmunology. 2013; 2(1). doi:10.4161/onci.22058
- Sotgia F, Whitaker-Menezes D, Martinez-Outschoorn UE, et al. Mitochondrial metabolism in cancer metastasis. Cell Cycle. 2012; 11(7): 1445-1454. doi:10.4161/cc.19841
- Alfonsi R, Grassi L, Signore M, et al. The Double Face of Exosome-Carried MicroRNAs in Cancer Immunomodulation. International Journal of Molecular Sciences. 2018; 19(4): 1183.
- Wu Y, Kwak KJ, Agarwal K, et al. Detection of Extracellular RNAs in Cancer and Viral Infection via Tethered Cationic Lipoplex Nanoparticles Containing Molecular Beacons. Analytical Chemistry. 2013; 85(23): 11265-11274.
- Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017; 8(34): 57813–57825.
- Ye SB, Li ZL, Luo DH, et al. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014; 5(14): 5439–5452.
- Ding G, Zhou L, Qian Y, et al. Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212-3p. Oncotarget. 2015; 6(30): 29877–29888.
- Shiga K, Hara M, Nagasaki T, et al. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers. 2015; 7(4): 2443–2458.
- De Wever O, Demetter P, Mareel M, et al. Stromal myofibroblasts are drivers of invasive cancer growth. International Journal of Cancer. 2008; 123(10): 2229-2238.
- Ringuette Goulet C, Bernard G, Tremblay S, et al. Exosomes Induce Fibroblast Differentiation into Cancer-Associated Fibroblasts through TGFβ Signaling. Molecular Cancer Research. 2018; 16(7): 1196-1204.
- Wei M, Yang T, Chen X, et al. Malignant ascites-derived exosomes promote proliferation and induce carcinoma-associated fibroblasts transition in peritoneal mesothelial cells. Oncotarget. 2017; 8(26): 42262–42271.
- Ning X, Zhang H, Wang C, et al. Exosomes Released by Gastric Cancer Cells Induce Transition of Pericytes Into Cancer-Associated Fibroblasts. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2018; 24: 2350–2359.
- Paggetti J, Haderk F, Seiffert M, et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015; 126(9): 1106–1117.
