Endocytosis and human innate immunity
Xiaofeng Ding1, Shuanglin Xiang1*
1Key Laboratory of Protein Chemistry and Development Biology of State Education Ministry of China, College of Life Science, Hunan Normal University, Changsha, P.R. China
Abstract
Endocytosis is critical for normal cellular function through clearing foreign materials and protecting the host from pathogen/virus attack. Innate immune cells play important roles in specifically recognizing and degrading microbes by generating phagosomes and phagolysosomes. However, the knowledge of how innate immunity regulates endocytosis in vitro and in vivo remains limited. In this review, we attempt to systematically and comprehensively summarize our current understanding of endocytosis and the role of Rab GTPases in the innate immune system. Understanding the immunity mechanisms of endocytosis might help develop targeted therapeutics for various applications, including viral inactivation and clearance, pathogen removal and even adjuvant-enhanced antibody responses.
Endocytosis
Endocytosis is a process in which a cell internalizes non-particulate materials such as proteins by engulfing them in an energy-dependent manner. Endocytosis generally includes pinocytosis (cell drinking), receptor-mediated endocytosis and phagocytosis (cell eating)1. Pinocytosis is fluid endocytosis of small particles suspended in extracellular fluid in most mammalian cells, and receptor-mediated endocytosis is ingestion of specific substances that bind to receptor on cell membrane in a clathrin-dependent pathway, while caveolae-, Arf-6, flotillin-1-, CDC42- and RhoA-dependent endocytosis is clathrin-independent2. Phagocytosis is generally defined as the uptake of particles (around 1µm or greater in diameter) including foreign pathogens, dead or dying cells, and other particulate debris3. Phagocytosis predominantly occurs in professional phagocytes such as macrophages (MΦs), monocytes and neutrophils. The endocytic pathway includes the specific binding of pathogen molecules to surface receptors on phagocyte and induction of actin polymerization, that drives to the material internalization to form a phagosome, fusion with late endosomes and lysosomes to sort them for degradation. Phagocytosis is a front-line defense against pathogen attack, and represents a vital facet of the innate immune response to pathogens4.
Endocytosis and the innate immune response
Endocytosis regulates microbial infection in autoimmune and inflammatory disease and is tightly associated with innate immune cells. Macrophages, neutrophils and dendritic cells (DCs) serve as critical phagocytes to drive the innate immune response. In addition, innate immune cells produce and release cytokines, which are critical responses to inflammation and infection5.
Macrophages
Macrophages, the principal tissue-resident effector cells of the innate immune system, express receptors to recognize foreign material such as pathogens and viruses6. Phagocytic receptors include Fc- and complement-receptors as well as receptors for particle- or pathogen-associated molecular patterns (PAMPs) such as Toll-like receptors (TLRs)7. Phagocytic uptake of particles by macrophage cells proceeds as follows: 1) access of particles to the surface of the macrophage membrane, 2) particle recognition by phagocytic receptors on the macrophage membrane, and 3) dynamic changes in membrane structure (protrusion or invagination) (Figure 1). Simultaneously, macrophages orchestrate the inflammatory response through pattern recognition receptor-mediated responses to avoid tissue damage8.
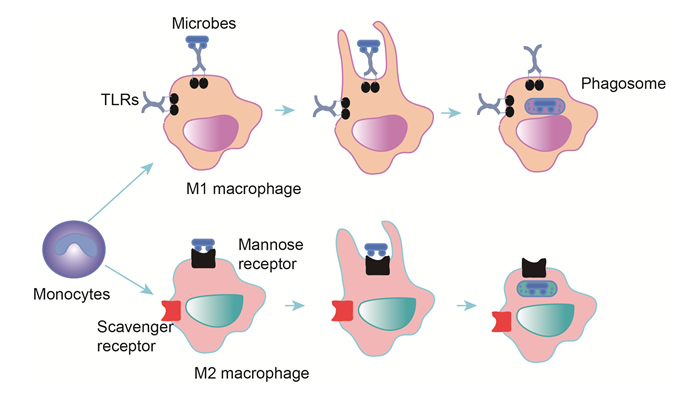
Figure 1: Phagocytosis in macrophages
Monocytes differentiate into M1 and M2 macrophages, there are different stimuli for two types-IFN? (M1), IL-4, IL-13 and IL-10 for M2. Macrophages express specific receptors for bacterial binding, which initiates the release of cytokines. Macrophages engulf and digest bacteria to form phagosome, and phagosome is fused with a lysosome to generate phagolysosomes. Microbes are killed by enzymes and other chemicals. Indigestible materials are discharged by exocytosis.
Macrophages were broadly divided into classically (M1) and alternatively (M2) activated. M1 activated macrophages are strongly positive for class II-MHC, present antigen to T lymphocytes that neutralize cells infected by microorganisms. The M1 macrophage is activated by interferon-?, which is produced by stimulated Th1 lymphocytes and Natural Killer (NK) cells9. The M2 macrophage is associated with Th2 activation and induced by IL-410. M1 macrophages have a lower endocytic ability compared to M2 macrophages, approximately 20% for M1 compared to 50-100% for M2 macrophages, with IL-10-stimulated M2 macrophages displaying the highest endocytic ability11, 12. M1 activation is associated with an increase in HIV-1 endocytosis whereas the opposite effect is observed in M2 macrophage subset (M2a). However, both processes are associated with increases in virus degradation and HIV-1 endocytosis13. But pathogens have evolved immune invasion mechanisms to survive, autophagy pseudomonas aeruginosa (PA) induced could reduce the formation of phagosomes and phagolysosomes, suppress bacterial internalization in macrophages14. Autophagy is a process of cellular autophagocytosis in times of nutritional stress. Autophagosomes in autophagy have many shared features with phagosomes but are unique. The autophagosome is controlled fusion of subcellular organelles with lysosomes to generate a double-membrane structure15.
Neutrophils and DCs
Neutrophils are very important innate immune cells comprising the first line of innate immune defense against infectious diseases16. Neutrophils arrive at sites of inflammation hours before monocytes. Neutrophils also express Fc- and complement-receptors like monocytes to facilitate phagocytic movement and kill ingested bacteria17. A neutrophil is equipped with two major pathways for killing: generation of Reactive Oxygen Species (ROS), and degranulation of granules packed with proteases and specific anti-microbial peptides. The active phagocytosis by neutrophils is eventually followed by the formation of neutrophil extracellular traps (NETs) in which externalized chromatin could entangle bacteria18. Upon recognition of opsonized Staphylococcus aureus, neutrophils internalize the bacterium in a phagosome where secretory granule content is released and ROS are produced19. Neutrophils exert mutual cooperation on other innate immune cells, make an important contribution in the activation and recruitment of macrophages at the site of infection or acute inflammation, and generate various chemotactic factors to activate DCs20, 21.
DCs represent a class of professional antigen-presenting cells whose primary function is to present antigens to the immune system, not to clear invading microorganisms as professional phagocytes. Mature DC lose phagocytic capacity and become potent antigen-presenting cells22. But immature DCs are highly phagocytic, efficiently and continuously sampling the antigenic content of their environment by phagocytosis of particulates. Unlike in normal tissues, DCs rather than macrophages with high MHC II levels are the major phagocytic cell in immune destruction and eventual regression of skin tumors23.
Other innate immune cells (Mast cells, NK cells, Gamma delta T cells and eosinophils)
In addition, there are other innate immune cells, including Mast cells (MCs), NK cells, Gamma delta T (γδT) cells and eosinophils (Eos). MCs are associated with wound healing and defense against pathogens. When activated, MCs detect pathogens and send danger signals and rapidly releasing characteristic granules and cytokines to promote pathogen-specific clearance and simultaneously recruit neutrophils and macrophages24. NK cells are a critical kind of cytotoxic lymphocyte to the innate immune system. NK cells recognize virus-infected cells dependent on activating KIR/Ly49 and NKG2D receptors25 and destroy compromised host cells known as “missing self cells”26. Epstein-Barr virus (EBV) infection is cleared in humans by strong NK cell and possible γδT cell responses27. γδT cells are an important subset of the innate immunity population of T cells in vivo, and account for 5-15% of T cells in peripheral blood. The largest subset of human γδ T cells is the Vγ2 (alternate Vγ9) Vδ2 subset, large numbers of Vγ9/Vδ2 T cells respond within hours to common molecules produced by microbes28. Eosinophilic phagocytosis is accompanied by degranulation and involves lysosomal enzymes, similar function to neutrophil cells. However, phagocytosis by eosinophils occurs less efficiently than neutrophil phagocytosis. Whereas the mechanism of phagocytosis by eosinophils was mediated by complement receptor 1 (CD35 ), that of neutrophils was modulated by CD16 and CD3229. Finally, these innate immune cells synergistically function to form the sentinels at the potential portals of microbial entry30 (Figure 2).
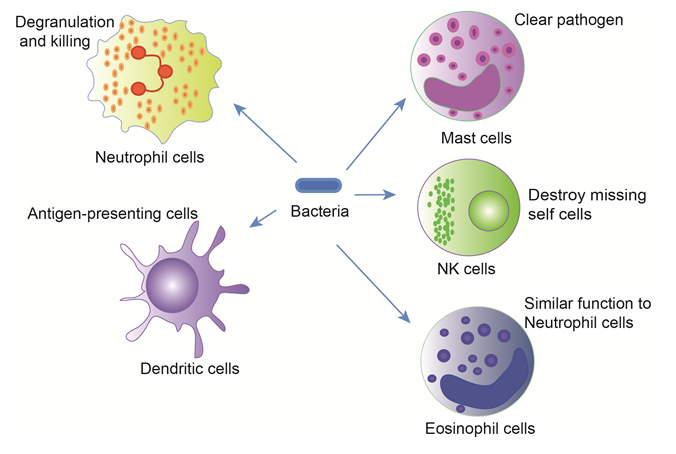
Figure 2: Phagocytosis in main innate immune cells
The innate immune cells include mast cells and natural killer cells, phagocytes (monocytes, macrophages and dendritic cells), and the granulocytes (neutrophils, eosinophils and basophils). Each cell type is equipped with different mechanisms to attack and eliminate pathogens from the host, but these innate immune cells could synergistically function to combat microbial entry.
Exosomes are a type of important membrane vesicles 30-100 nm in diameter and contain various molecular constituents including protein, mRNA and miRNA, transfer molecules from one cells to another via membrane vesicle trafficking, thereby influencing the immune system31. Exosomes enter phagocytic cells via phagocytosis, move to phagosomes and further sort into phagolysosomes, in an actin- and phosphatidylinositol 3-kinase (PI3K)-dependent way32. Exosomes released from HBV-infected hepatocytes stimulated NK cells and macrophages in innate immune response against HBV33. Exosomes derived from bacterially infected macrophages could stimulate macrophages and neutrophils to secrete proinflammatory mediators, and DCs-derived exosomes are also powerful immunoregulators34. Hence, the exosome uptake through phagocytosis plays important roles in exosome-cell interactions and intracellular trafficking pathway, and shows new light on a novel class of drug delivery systems.
Interplay between endocytosis proteins Rab and innate immunity
About 20 Rab proteins among over 70 mammalian Rab GTPases are regulators of endocytosis. The Rab5 subfamily (Rab5, Rab21, and Rab22) are localized to early endosomes and plasma membrane and regulate early endocytosis and signal transduction. Rab5 has a critical role in controlling both phagosome-early endosome fusion and the maturation of phagosomes into degradative compartments. In one respect, IL4 and 6 upregulate Rab5 expression35. IL-4 also prolongs the retention of Rab5 on phagosomes in a PI3K-dependent manner36. In the other respect, macrophages stimulated with IFN-γ show an increase of Rab5a and partner Rab20 expression in phagosome maturation37, 38. Moreover, IFN-γ produced by NK and NKT cells is critical for combating viral and bacterial infections and contributes to macrophage activation by increasing phagocytosis and producing cytokines39. Rab22 recruits Rabex-5 (a Rab5 GDP/GTP exchange factor (GEF)) to early endosomes for activation of Rab5 and stimulation of early endosome fusion40. Rab proteins interact with different effectors and regulators, and RABEP1 (Rab GTPase-binding effector protein 1) functions as a vital regulator and molecular switch for Rab5 function. We previously found that Intersectin 2 long isoform (ITSN2L) interacts with RABEP1 and stimulates the degradation of RABEP1 to regulate endocytosis and endosome trafficking41. Therefore, we speculate that cytoskeletal protein ITSN2L might regulate central Rab5 by inhibiting the function of regulator RABEP1 to interfere with early endocytosis and innate immune response, which will be further investigated in our future studies.
The early endosome segregate molecules are sorted to plasma member through fast (Rab4, Rab14, Rab15) and slow (Rab11a, Rab15, Rab22a) recycling routes42. GTPase subsets (Rab14, Rab20, Rab22a, Rab32, Rab34, Rab38, Rab39, Rab43) interact to regulate phagosome formation42, 43. Rab32 formed a persistent complex with two interacting proteins, Prohibitin (PHB) and PHB2, to encompass bacteria during early phagosome formation44. Rab7 and Rab34 regulates the fusion with late endocytic compartments in late phagosomes45. IL-12 is naturally produced by DCs, macrophages, neutrophils, and human B-lymphoblastoid cells in response to antigenic stimulation46. IL-12 then upregulates Rab7 expression by activating p38 mitogen-activated protein kinase (p38 MAPK) in intracellular trafficking47. Taken together, a network of Rab cascades regulate certain critical steps of innate immune responses. Rab5 and Rab7 are the best characterized Rab proteins in endocytosis (Figure 3), the way that most of GTPases function in pathogen control by immune cells remains poorly defined.
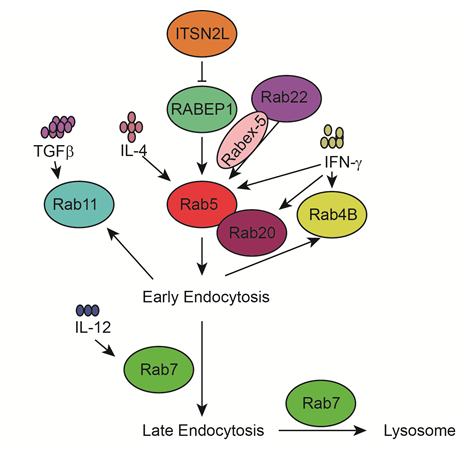
Figure 3: Rab5/7 proteins and immune factors in endocytosis
Early endocytosis is mainly regulated by Rab5. RABEP1 functions as a vital regulator for Rab5. ITSN2L interacts with RABEP1 and stimulates RABEP1 degradation. Rab22 recruits Rabex-5 to early endosomes for activation of Rab5. IFN-γ increases the expression of Rab5 and partner Rab20 in phagosome maturation. Indigestible molecules can return to the plasma membrane via fast (Rab4B) or slow (Rab11) recycling routes. Late endosome and phagosome fusion with lysosomes is promoted by another critical Rab GTPase, Rab7, which is induced by IL-12.
Perspectives
Endocytosis has been studied for over a century since Metchnikoff put it forward in 1882. Thus, it has become clear that endocytosis regulates normal cell physiology linked with important innate immune responses. But many aspects are not yet well described. Especially, (1) to understand many disease pathology it is necessary to know more about the basic cell biology of endocytosis. The dynamics interplay between basic science and disease-targeted research is expected to yield new findings and novel therapies in future. (2) More studies are still required to define endocytosis using genomics and proteomics techniques, and high-throughput screen signal pathways in Rab cascades and innate immune signaling to identify specific targets and interactions for important innate immune responses and immune evasion of many pathogens. (3) With the rapid development of advanced technology, it is possible to intensively investigate endocytosis from in vitro studies to in vivo models. There are only a few examples about the effect of the immune-regulatory role on endocytosis in vivo48, 49. We expect that a better understanding of the regulation of endocytosis on innate immune response may provide a basis for the development of immunotherapeutic approaches to design new drugs or approved existing drugs to disrupt pathogen growth or infection and inhibit chronic viral infections.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81770389 & No. 81272190), Distinguished Youth Foundation of Hunan Province (No. 2015JJ1011), State Key Laboratory of Developmental Biology of Freshwater Fish, and the Cooperative Innovation Center of Engineering and New Products for Developmental Biology of Hunan Province (20134486).
There is no conflict of interest.
References
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem.2009; 78: 857-902.
- Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, et al. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release.2017; 266: 100-8.
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature.2003; 422 (6927): 37-44.
- Greenberg S, Grinstein S. Phagocytosis and innate immunity. Curr Opin Immunol.2002; 14 (1): 136-45.
- Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood.2011; 118 (1): 9-18.
- Davies LC, Taylor PR. Tissue-resident macrophages: then and now. Immunology.2015; 144 (4): 541-8.
- Janeway CA, Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol.2002; 20: 197-216.
- Cole J, Aberdein J, Jubrail J, et al. The role of macrophages in the innate immune response to Streptococcus pneumoniae and Staphylococcus aureus: mechanisms and contrasts. Adv Microb Physiol.2014; 65: 125-202.
- Schroder K, Hertzog PJ, Ravasi T, et al. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol.2004; 75 (2): 163-89.
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol.2003; 3 (1): 23-35.
- Edin S, Wikberg ML, Rutegard J, et al. Phenotypic skewing of macrophages in vitro by secreted factors from colorectal cancer cells. PLoS One.2013; 8 (9): e74982.
- Tarique AA, Logan J, Thomas E, et al. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am J Respir Cell Mol Biol.2015; 53 (5): 676-88.
- Gobeil LA, Lodge R, Tremblay MJ. Differential HIV-1 endocytosis and susceptibility to virus infection in human macrophages correlate with cell activation status. J Virol.2012; 86 (19): 10399-407.
- Wu Y, Li D, Wang Y, et al. Pseudomonas aeruginosa promotes autophagy to suppress macrophage-mediated bacterial eradication. Int Immunopharmacol.2016; 38: 214-22.
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell.2004; 6 (4): 463-77.
- Kaufmann SH. Immunology's foundation: the 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nat Immunol.2008; 9 (7): 705-12.
- Kim MK, Huang ZY, Hwang PH, et al. Fcgamma receptor transmembrane domains: role in cell surface expression, gamma chain interaction, and phagocytosis. Blood.2003; 101 (11): 4479-84.
- Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol.2012; 189 (6): 2689-95.
- Lu T, Porter AR, Kennedy AD, et al. Phagocytosis and killing of Staphylococcus aureus by human neutrophils. J Innate Immun.2014; 6 (5): 639-49.
- Kasama T, Strieter RM, Standiford TJ, et al. Expression and regulation of human neutrophil-derived macrophage inflammatory protein 1 alpha. J Exp Med.1993; 178 (1): 63-72.
- Ethuin F, Gerard B, Benna JE, et al. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab Invest.2004; 84 (10): 1363-71.
- Nagl M, Kacani L, Mullauer B, et al. Phagocytosis and killing of bacteria by professional phagocytes and dendritic cells. Clin Diagn Lab Immunol.2002; 9 (6): 1165-8.
- Byrne SN, Halliday GM. Phagocytosis by dendritic cells rather than MHC IIhigh macrophages is associated with skin tumour regression. Int J Cancer.2003; 106 (5): 736-44.
- St John AL, Abraham SN. Innate immunity and its regulation by mast cells. J Immunol.2013; 190 (9): 4458-63.
- Hamerman JA, Ogasawara K, Lanier LL. NK cells in innate immunity. Curr Opin Immunol.2005; 17 (1): 29-35.
- Lakshmikanth T KK, Sreerama K . The Natural Killer Cell - 'Missing-Self' Recognition Strategy. WebmedCentral IMMUNOLOGY.2011; 2 (8): WMC002068.
- Djaoud Z, Guethlein LA, Horowitz A, et al. Two alternate strategies for innate immunity to Epstein-Barr virus: One using NK cells and the other NK cells and gammadelta T cells. J Exp Med.2017; 214 (6): 1827-41.
- Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol.2006; 126 (1): 25-31.
- Hatano Y, Taniuchi S, Masuda M, et al. Phagocytosis of heat-killed Staphylococcus aureus by eosinophils: comparison with neutrophils. APMIS.2009; 117 (2): 115-23.
- Hoffmann JA, Kafatos FC, Janeway CA, et al. Phylogenetic perspectives in innate immunity. Science.1999; 284 (5418): 1313-8.
- Li XB, Zhang ZR, Schluesener HJ, et al. Role of exosomes in immune regulation. J Cell Mol Med.2006; 10 (2): 364-75.
- Feng D, Zhao WL, Ye YY, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic.2010; 11 (5): 675-87.
- Kouwaki T, Fukushima Y, Daito T, et al. Extracellular Vesicles Including Exosomes Regulate Innate Immune Responses to Hepatitis B Virus Infection. Front Immunol.2016; 7: 335.
- O'Neill HC, Quah BJ. Exosomes secreted by bacterially infected macrophages are proinflammatory. Sci Signal.2008; 1 (6): pe8.
- Wainszelbaum MJ, Proctor BM, Pontow SE, et al. IL4/PGE2 induction of an enlarged early endosomal compartment in mouse macrophages is Rab5-dependent. Exp Cell Res.2006; 312 (12): 2238-51.
- de Keijzer S, Meddens MB, Kilic D, et al. Interleukin-4 alters early phagosome phenotype by modulating class I PI3K dependent lipid remodeling and protein recruitment. PLoS One.2011; 6 (7): e22328.
- Alvarez-Dominguez C, Stahl PD. Interferon-gamma selectively induces Rab5a synthesis and processing in mononuclear cells. J Biol Chem.1998; 273 (51): 33901-4.
- Pei G, Schnettger L, Bronietzki M, et al. Interferon-gamma-inducible Rab20 regulates endosomal morphology and EGFR degradation in macrophages. Mol Biol Cell.2015; 26 (17): 3061-70.
- Prashar A, Schnettger L, Bernard EM, et al. Rab GTPases in Immunity and Inflammation. Front Cell Infect Microbiol.2017; 7: 435.
- Chavrier P, Parton RG, Hauri HP, et al. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell.1990; 62 (2): 317-29.
- Yang X, Yan F, He Z, et al. ITSN2L Interacts with and Negatively Regulates RABEP1. Int J Mol Sci.2015; 16 (12): 28242-54.
- Agola JO, Jim PA, Ward HH, et al. Rab GTPases as regulators of endocytosis, targets of disease and therapeutic opportunities. Clin Genet.2011; 80 (4): 305-18.
- Deretic V. Autophagosome and phagosome. Methods Mol Biol.2008; 445: 1-10.
- Li Y, Wang Y, Zou L, et al. Analysis of the Rab GTPase Interactome in Dendritic Cells Reveals Anti-microbial Functions of the Rab32 Complex in Bacterial Containment. Immunity.2016; 44 (2): 422-37.
- Colucci AM, Campana MC, Bellopede M, et al. The Rab-interacting lysosomal protein, a Rab7 and Rab34 effector, is capable of self-interaction. Biochem Biophys Res Commun.2005; 334 (1): 128-33.
- Kalinski P, Hilkens CM, Snijders A, et al. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol.1997; 159 (1): 28-35.
- Bhattacharya M, Ojha N, Solanki S, et al. IL-6 and IL-12 specifically regulate the expression of Rab5 and Rab7 via distinct signaling pathways. EMBO J.2006; 25 (12): 2878-88.
- Chen K, Nishi H, Travers R, et al. Endocytosis of soluble immune complexes leads to their clearance by FcgammaRIIIB but induces neutrophil extracellular traps via FcgammaRIIA in vivo. Blood.2012; 120 (22): 4421-31.
- Li SX, Barrett BS, Heilman KJ, et al. Tetherin promotes the innate and adaptive cell-mediated immune response against retrovirus infection in vivo. J Immunol.2014; 193 (1): 306-16.
