Pathological Approach to A Special Benign Tumor with Regard to its Differential Diagnose Spectrum: Intranodal Palisaded Myofibroblastoma
Yasemin Yuyucu Karabulut*
Mersin University Medical School, Department of Pathology, Mersin, Turkey
Abstract
Intranodal palisaded myofibroblastoma, also known as “intranodal hemorrhagic spindle cell tumor with amianthoid fibers,” is a benign mesenchymal tumor of the lymph node originating from smooth muscle cells and myofibroblasts often with the presence of amianthoid fibers. Ninety-three cases of intranodal palisaded myofibroblastoma have been reported in the literatüre since its first description and most of them have the same clinical history “painless firm nodüle”. It is mostly seen in inguinal region there are few cases have been described in other locations. It’s large and important differential diagnostic spectrum makes this tumor special.
Introduction
Intranodal palisaded myofibroblastoma (IPM) is a rare benign mesenchymal neoplasm with myofibroblastic smooth muscle differentiation, and collagen fibers known as amianthoid fibers1. It was first dercribed in 1989 and was named in different forms such as “palisaded myofibroblastoma”, “intranodal hemorrhagic spindle-cell tumor with amianthoid fibers” and “myofibroblastoma”1, 2.
In fact it’s a benign lesion which have no clinical importance, but in the literature, there is a case of sharing facts in precious medical journals. Because It is important in terms of the antities that make differential diagnosis such as schwannoma, kaposi sarcoma, hemangioendothelioma, dendritic reticulum cell tumor, and inflammatory myofibroblastic tumor (IMT)1–3. Also spindle-cell proliferation to lymph nodes is frequently seen as metastases from malignant melanoma, carcinoma with a pseudosarcoma feature or sarcoma.
Clinical Features
IPM commonly affects the second to eighth decade, with a peak incidence in the group between 40 and 60 years of age, but an infant patient has also been reported3. It is more common in men with a male to female ratio of 1.5. Usually IPM arises within inguinal lymph nodes. In the literatüre there are few cases affected cervical, axillary, and submandibular lymph nodes4-6. IPM presents as a solitary asymptomatic painless nodular mass. The tumor is well capsulated and usually involves a single lymph node without infiltrating the overlying skin4. But in the published data there are multiple and bilateral forms3,7. For example I had a case with the pre-diagnosis of lymphoma, who has multiple and bilateral inguinal lymph nodes7.
Macroscopic and Microscopic Features
Gross features include gray white tumor with multifocal hemorrhagic areas. It is usually possible to identify a rim of residual node at the periphery of the tumor (Figure 1(a, b)). Microscopically, the tumor consists of areas of low proliferative spindle cells, that have little pleomorphism, arranged in short intersecting or crisscrossed fascicles with foci voguely palisaded nuclei. In some areas, the cells form sheets with slit-like extracellular spaces containing eryhrocytes, that make it similar to kaposi sarcoma. Hemosiderin-laden histiocytes can be seen anywhere (Figure 2(a-d)). Metaplastic bone formation and calcification have also been reported4. In fact the tumor is benign and local excision is adequate and curative.
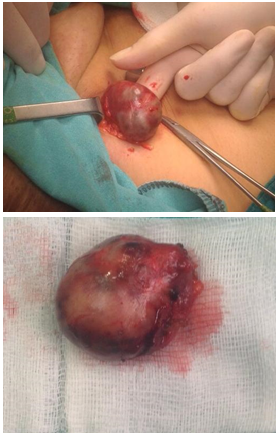
Figure 1(a,b). Large firm nodüler mass.
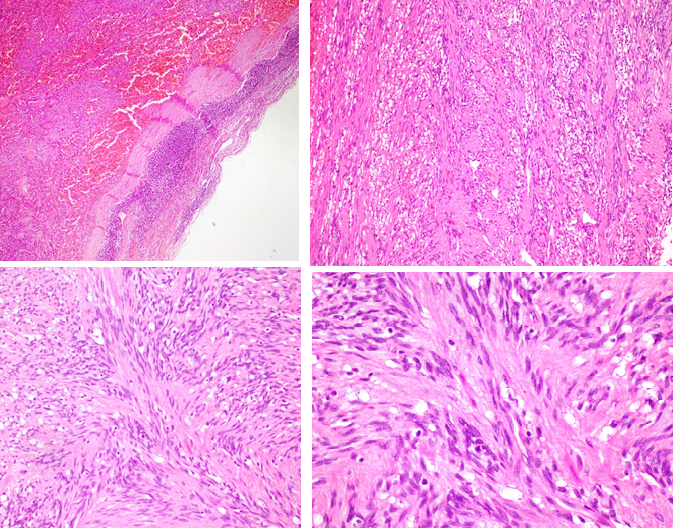
2(a). Remnant lymph node is seen at the periphery as a rim with extensive areas of fresh and old hemoragies with hemosiderin-laden macrophages within the tumor (H&Ex100).
2(b,c).The tumor consisted of bland spindle cells arranged in whorls and fascicles. b; (H&Ex100), c; (H&Ex200).
2(d). Low proliferative spindle cells, with little pleomorphism ; (H&Ex400).
Ninety-three cases have been reported in the literature so far. As I said before most of them affect inguinal lymph nodes. Most of them have same clinical history, such as painless firm nodüle.
Histology and Anchillary Methods
The histogenesis is still not completely understood. The cell of origin is most likely to be the myofibroblast or smooth muscle cell of the lymph node blood vessels1. The fact that inguinal lymph nodes, which are preferably involved, have richer vascular components supports this claim. Positive immunohistochemistry (IHC) for vimentin and actin, and negative for desmin supports the origin (Figure 3(a-d)). Electron microscopy shows irregular contours of the nucleus and occasional intra-nuclear pseudoinclusions.
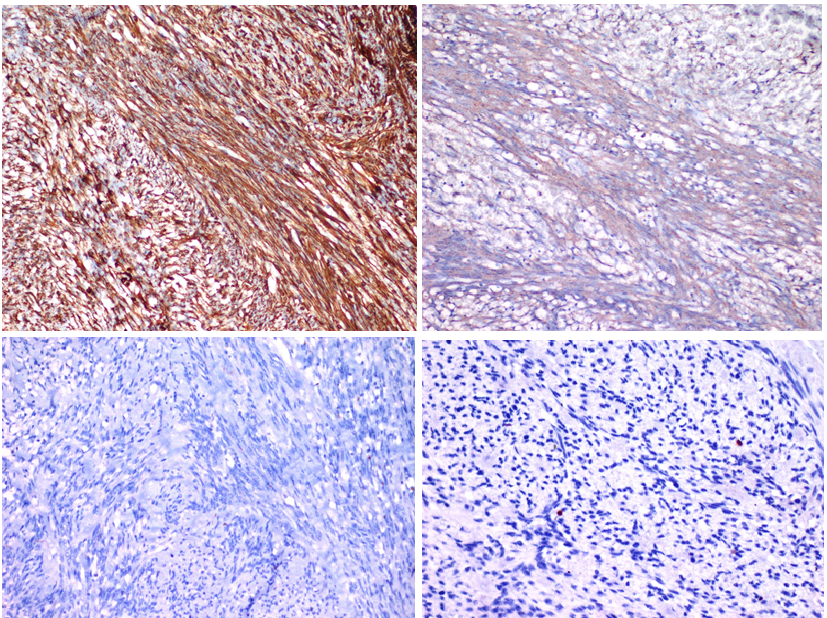
3a. Strong cytoplasmic positivity with vimentin (Vimentin x100).
3b. Cytoplasmic positivity with smooth muscle actin (SMAx200).
3c. Negatif with Desmin (Desmin x200)
3d. Low proliferatif index (Ki67 x 400)
Cytoplasm shows focal densities believed to be smooth muscle myofilaments9. Although having so little study on this topic, genetic evidence associated with HHV-8 and Epstein - Barr virus (EBV) suggests that a viral mechanism of oncogenesis might be involved in the development of these lesions9, 10. Kleist et al demonstrated cyclin D1 expression in 50% of spindle cells in these lesions11. Genetic studies conducted with the same cases did not show any mutations in the CCND1 gene locus and they suggested that cyclin D1 over-expression develops independently from any genetic changes and influences the spindle cells as a growth factor11. The most distinctive feature of the tumor is the “amianthoid” fibers or thick collagen mats which are nearly always present (Figure 4(a,b)). Nature of amianthoid fibers has been intriguing ever since, and an old study had an explanation like this; the amianthoid fibers are sometimes centered by small vascular vessels, and this finding has led some investigators to believe that the formation of these structures probably represents a degenerative process of perivascular collagen, perhaps linked to trauma or anoxia12. Anyway recent studies indicates that the center of these fibers is composed of type I collagen and type III collagen localized peripherally. Electron microscopy also confirms that amianthoid fiber is a collagen fiber and not an amianthoid fiber. This fiber has smaller width than amianthoid fiber ranging from 80 to 150 nm vs 280 to 1000 nm8.
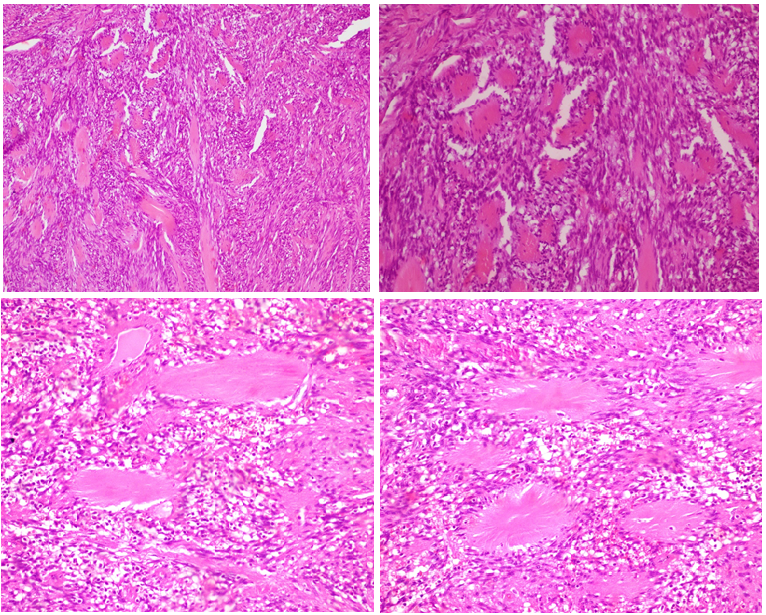
Figure 4(a-d). Among the spindle cells, ‘amianthoid fibers’ exhibiting an irregular distribution and forming stellate structures (H&Ex100), (H&Ex400).
Differential Diagnose
This tumor has a large pathologic differential diagnose spectrum, that develop from spindle cells. Malign and benign spindle-cell tumors and benign mesenchymal lesions of the lymph node should keep in mind. Neoplasms that have spindle-cell morphology can originate from vascular endothelium, fibroblasts, histiocytes, smooth muscle cells, myofibroblasts, dendritic reticulum cells, or interdigitating reticulum cells, all of which are stromal components of lymph nodes, are rarely seen lesions and they can imitate IPM histologically.
Schwannoma and KS are the most important tumors in the differential diagnosis. Moreover, hemangioendothelioma, dendritic reticulum cell tumor, and inflammatory myofibroblastic tumor (IMT) should also be considered in the differential diagnosis1-4, 10. Schwannomas, extending through the peripheral nerve sheath, contain spindle cells with nuclear palisading and are often confused with IPM. Since it lacks peripheral nerve innervation, the lymph node is not expected to be a site of schwannoma development. Immunohistochemically, detection of S-100 immunoreaction contributes to the differential diagnosis of those two lesions. Because IPM contains extravasated erythrocytes and hemosiderin pigments among the spindle cells, it may be mistaken for KS. In some IPM cases, detection of HHV-8 immunoreactivity reduces the utility of this marker in the differential diagnosis. However, histomorphologically, nuclear palisading and amianthoid fibers are not observed in KS. Moreover, the absence of immunoreaction with endothelial markers also supports an IPM diagnosis13,14.
Conclusion
Diagnosis of IPM is important since potential confusion with primary or metastatic mesenchymal neoplasms in the lymph nodes can be avoided. The clinical history, the physical examination, and the typical histological characteristics help in the correct diagnosis of IPM. Better understanding of IPM will help in proper diagnosis and management because IPM is a benign lesion with very low or negligible rate of recurrence. Regarding treatment, total removal of the lesion suffices.
References
- Weiss SW, Gnepp DR, Bratthauer GL. Palisaded myofibroblastoma: a benign mesenchymal tumor of lymph node. Am J Surg Pathol. 1989; 13(5): 341-346.
- Lee JY, Abell E, Shevechik GJ, Solitary spindle cell tumor with myoid differentiation of the lymph node. Arch Pathol Lab Med. 1989; 113(5): 547-550.
- Rahimi S, Muda AO, Faraggiana T. “Multicentric intranodal myofibroblastoma in an infant”. Histopathology. 1995; vol. 27, no. 5: 477-478.
- CreagerAJ Garwacki CP. “Recurrent intranodal palisaded myofibroblastoma with metaplastic bone formation”. Archives of Pathology and Laboratory Medicine. 1999; vol. 123, no. 5: 433–436.
- Lioe TF, Allen DC, Bell JC. “A case of multicentric intranodal palisaded myofibroblastoma”. Histopathology. 1994; vol. 24, no. 2: 173–175.
- Bhullar JS, Herschman BR, Dubay L. “Intranodal palisaded myofibroblastoma: a new entity of axillary tumors”. American Surgeon. 2013; vol. 79, no. 1: E19–E21.
- Karabulut Y, Kara T, Berke?o?lu M. Intranodal palisaded myofibroblastoma – a rare case report and literature review. APMIS. 2016; 124: 905–910.
- Michal M, Chlumska A, Skalova A et al. Palisaded intranodal myofibroblastoma. Electron microscopic study. Zentralbl Pathol. 1993; 139(1): 81-88.
- Cosenza UM, Galati G, Zofrea P. Clinical and biological features of an intranodal palisaded myofibroblastoma. Anticancer Res. 2006; 26: 2349-2352.
- Nguyen T, Eltorky MA. Intranodal palisaded myofibroblastoma. Arch Pathol Lab Med. 2007; 131: 306-310.
- Kleist B, Poetsch M, Schmoll J. Intranodal palisaded myofibroblastoma with overexpression of cyclin D1. Arch Pathol Lab Med. 2003; 127: 1040-1043.
- Hisaoka M, Hashiomoto H, Daimaru Y. lntranodal palisaded myofibroblastoma with so-called amianthoid fibers: A report of two cases with a review of the literature Pathology lnternational. 1998; 48: 307-312.
- Abd el-All HS. Breast spindle cell tumours: about eight cases. Diagn Pathol. 2006; 22: 113.
- Ciralik H, Ezberci F, Bulbuloglu E, et al. Intranodal palisaded myofibroblastoma and differential diagnosis: a case report. Chin Med J. 2005; 18: 1758–60.
