Targeted IL-15-based Protein Fusion Complexes as Cancer Immunotherapy Approaches
Sarah Alter1, Peter R. Rhode1, Emily K. Jeng1, and Hing C. Wong1*
1Altor BioScience, Miramar, Florida
Abstract
This mini review provides an overview and rationale for creating IL-15-based fusion protein complexes to be used as targeted immunotherapeutic agents. IL-15 stimulates proliferation and activation of CD8+ T and natural killer cells which result in augmentation of their anti-tumor activities. We have created ALT-803, an IL-15 superagonist complex which exhibits longer serum half-life, longer retention in lymphoid tissues, and better immunostimulatory and anti-tumor activities compared to native IL-15. When used alone or in combination with other immunotherapeutic molecules in various mouse tumor models, ALT-803 effectively reduces tumor burden and prolongs survival by stimulating the innate and adaptive arms of the immune system. To evaluate whether ALT-803 could be used as a protein scaffold to create IL-15-based tumor cell-specific molecules, we genetically fused it with a single chain anti-CD20 antibody derived from the variable regions of rituximab. This novel fusion protein exhibits enhanced anti-tumor activity compared to rituximab while maintaining IL-15 immunostimulating properties. Thus, ALT-803 may be exploited as a versatile scaffold to produce multivalent targeted fusion proteins to improve the anti-tumor efficacy of other therapeutic agents in the clinic.
Interleukin-15 and ALT-803
Common gamma chain (γc) cytokines, which include interleukin (IL)-2, IL-4, IL-7, IL-9, IL-15, and IL-21, have been studied extensively and shown to be promising as cancer immunotherapeutic agents1. Recombinant IL-2 (rIL-2) has been approved for treatment of patients with metastatic renal cell carcinoma and melanoma more than two decades ago. Although rIL-2 can induce durable and major responses in a subset of patients2,3, the use of rIL-2 at the effective dose level causes severe side effects, such as capillary leak syndrome and hypertension, requiring extensive in-patient care during its administration4. These treatment-induced side effects curtail the clinical utilities of rIL-2 as an immunotherapeutic drug.
IL-15, a four helix γc cytokine, is structurally related to rIL-25. The two γc cytokines use the same IL-2/IL-15 receptor βγc (IL-2/15Rβγc) displayed on the surface of natural killer (NK) and T cells for signaling1. However, IL-15 has been proven to stimulate anti-tumor immune responses of NK and T cells without the induction of IL-2-associated capillary leak syndrome, activation-induced cell death, and expansion of T regulatory cells1,6. Thus, IL-15 is considered a promising immunotherapeutic for cancer treatment3. IL-15 associates with IL-15 receptor α (IL-15Rα) on monocytes and dendritic cells and is trans-presented to form a complex with the IL-2/15Rβγc on NK and T cells resulting in their activation5,7. To develop an IL-15-based therapeutic agent, we created a soluble complex, ALT-803, consisting of two protein subunits of a human IL-15 variant with an asparagine to aspartic acid substitution at position 72 (N72D) associated to a dimeric human IL-15Rα sushi (IL-15RαSu) domain/human IgG1 Fc fusion protein (IL-15RαSuFc) (Figure 1A)8,9. The N72D mutation increases the binding affinity of IL-15 to the IL-2/15Rβγc complex approximately 4- to 5-fold and significantly enhances the IL-15 biological activities8. Compared to native IL-15, ALT-803 exhibits increased in vivo half-life (25 hours versus <40 minutes for native IL-15)9 and better distribution to the lymphoid organs (i.e., lymph nodes, spleen) for prolonged immune cell stimulation10. ALT-803 is capable of simultaneously activating innate and adaptive immune cells and eliciting both rapid and long lasting anti-tumor activity as well as providing protective anti-tumor immunity10-19. In pre-clinical models, systemic ALT-803 administration leads to antigen-independent proliferation, activation, and differentiation of memory CD8+CD44high T cells into potent effector cells and upregulation of NKG2D (KLRK1), but not CD25 or PD-110,11,13,14,17. These CD8+ T cells exhibit increased cytotoxic activity against tumor cells, as shown by secretion of high levels of IFNγ, granzyme B, and perforin10,11. In addition to T cell stimulation, ALT-803 also induces NK cell proliferation, degranulation (measured by CD107a expression), IFNγ and TNFα production, and tumor cell killing10,12,13,15,17-19. ALT-803 was also shown to promote robust expansion of NK cells with the highest fold increase observed in “high effector” CD11b+CD27high NK cells which are resistant to self-tolerance, are highly cytotoxic, and exhibit increased cytokine production and migratory capacity13. Felices et al. demonstrated that ALT-803 can restore function of ovarian cancer patient ascites-derived NK cells and enhance cytotoxicity against ovarian tumor cells in vitro and in vivo18. In non-human primates, ALT-803 treatment results in increased peripheral blood CD4+, CD8+ T and NK cell levels following four consecutive weekly doses with no significant treatment-dependent adverse effects10.
Another mechanism by which ALT-803 enhances NK cell-mediated lysis of tumor cells is through antibody-dependent cellular cytotoxicity (ADCC)15,20. Short-term ALT-803 stimulation of human NK cells in vitro was shown to enhance rituximab-directed ADCC against Daudi and Raji tumor cell lines. ALT-803 combination treatment with rituximab in B-cell lymphoma-bearing mice results in significant reduction of tumor burden and increased survival when compared to treatment with rituximab alone15. Additionally, when combined with other potential immunotherapeutic molecules, such as checkpoint inhibitors, NKG2D ligand neutralizing antibody, or MEK inhibitor, ALT-803 improves the efficacy and durability of anti-tumor responses12-14,16,20. Combining the immunostimulatory property of ALT-803 with the blockade of inhibitory immune checkpoints in multiple in vivo tumor models has been shown to increase the therapeutic effect of various checkpoint inhibitors (i.e., PD-1, PD-L1, and CTLA-4) by promoting development and function of effector NK and CD8+ T cells and mediating a more potent anti-tumor activity versus checkpoint inhibition alone13,14. Therefore, although ALT-803 exhibits anti-tumor efficacy as a monotherapy, it also has great potential for use in combination with other immunotherapeutic agents. Importantly, mice cured by ALT-803 treatment have been shown to be resistant to tumor rechallenge, which indicates that ALT-803 also induces long-term memory and durable anti-tumor immunity11,14,16. As a result, ALT-803 is being developed as a potential cancer immunotherapeutic and is currently in multiple clinical trials for treatment of patients with advanced solid and hematologic malignancies.
ALT-803-based Protein Scaffold for Targeted Immunotherapeutics
ALT-803 may act as a versatile protein scaffold, which can be modified for the creation of novel target-specific immunotherapeutic agents8,21,22. We previously showed that IL-15 binding and function are preserved when ALT-803 components are fused to single chain T cell receptor (TCR) antigen binding domains21. These targeted fusion proteins consist of TCRs recognizing a peptide of human p53 protein (c264scTCR or c149scTCR), or an OVA protein epitope (OT1scTCR), linked to either IL-15, IL-15N72D, or IL-15RαSu N termini8,21. Dimerization through IL-15:IL-15Rα or IL-15N72D:IL-15Rα interaction was shown to maintain the individual TCR’s functional activity and increase effective antigen binding through enhanced avidity21. Additionally, TCR fusion to N termini of either IL-15:IL-15RαSu or IL-15N72D:IL-15RαSu scaffold provides the spatial orientation necessary for functionally independent heterodimer complexes while retaining flexibility to allow folding of closely paired TCR chains21. These fusion proteins are readily produced and easily purified from cell culture supernatant at a relatively high level. Since ALT-803 is comprised of an IL-15N72D:IL-15RαSuFc dimer, the results of the studies discussed above suggest that ALT-803 can be used to generate multivalent disease-specific fusion proteins (Figure 1).
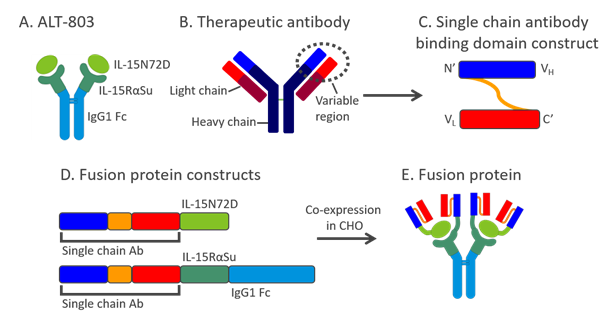
Figure 1: ALT-803 as a scaffold for creation of novel fusion molecules
A schematic diagram showing construction of novel fusion molecules using ALT-803 as a scaffold and a therapeutic antibody as an example. A. ALT-803 structure:IL-15N72D:IL-15RαSuFc complex consisting of IL-15N72D associated with the dimeric IL-15RαSuFc fusion protein. B. Structure of a therapeutic antibody showing the variable region consisting of the light and heavy chains. C. Variable gene segment of the antibody light chain linked to the 5’ end of the variable gene segment of the antibody heavy chain via a Gly4Ser3 linker to create a single chain antibody. D. A single chain antibody construct genetically fused to the 5’ end of the IL-15N72D mutein and a single chain antibody construct genetically fused to the 5’ end of the IL-15RαSuFc construct. E. A soluble novel ALT-803-based fusion molecule is produced. The fusion protein is comprised of four single chain antibody domains: two are fused to the two IL-15N72D muteins and two are fused to the two IL-15RαSuFc fusion constructs.
Based on previous data demonstrating that ALT-803 can enhance rituximab’s anti-tumor activity, we created 2B8T2M, a novel CD20-specific fusion protein complex. Four single chain domains of the monoclonal antibody, rituximab, were genetically fused to N termini of IL-15N72D and IL-15RαSuFc proteins22. This molecule exhibits tri-specific binding activity through its recognition of CD20 on B lymphoma cells, IL-2/15Rβγc on immune cells, and Fc receptor on NK cells and macrophages (Figure 2). When cultured with Daudi tumor cells, 2B8T2M shows enhanced pro-apoptotic activity that is comparable to >600-fold higher concentration of rituximab alone and is dependent on CD20 binding. Additionally, 2B8T2M is significantly more effective at inducing ADCC against Daudi tumor cells by human immune cells compared to rituximab22. A biodistribution study of the fusion protein demonstrated that adding a targeting domain to ALT-803 does not alter the molecule’s ability to home to lymphoid tissues and be retained there for at least 70 hours22. In Daudi lymphoma-bearing mice, 2B8T2M treatment results in significantly lower tumor burden and improved survival compared to rituximab treatment alone. Specific B cell depletion by 2B8T2M was also observed in cynomolgus monkeys and importantly, no significant adverse events were observed22. These results demonstrate that fusing a single chain antibody domain to ALT-803 is an effective strategy to potentiate tumor targeting immunotherapeutic molecule without compromising its immunostimulatory capabilities. Based on these results, 2B8T2M is currently in late-stage pre-clinical development.
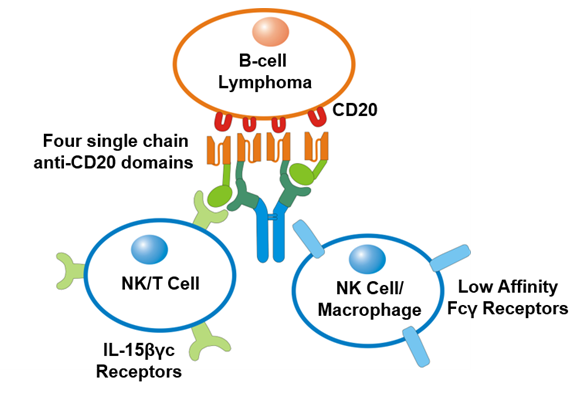
Figure 2: 2B8T2M exhibits tri-specific binding activity
2B8T2M, a fusion protein with four single chain anti-CD20 Ab domains fused to ALT-803, is shown to recognize and specifically bind the CD20 molecule on a B-cell lymphoma cell, as well as low affinity Fcγ receptors on an NK cell or macrophage. Also shown is binding of the IL-15N72D:IL-15RαSu portion of 2B8T2M to IL-2/15Rβγc on NK and T cells for immune stimulation.
Generating a single agent with multiple tumor targets and anti-tumor activities may be more beneficial compared to a combination of individual molecules for treatment of cancer. Our data show that linking a CD20 targeting antibody to ALT-803 retains its superior IL-15 activity while mediating more specific tumor killing22. In addition to improved anti-tumor efficacy, the ALT-803 scaffold may also provide a vehicle for preferential delivery to lymphoid organs to allow proper and prolonged immune activation22.
Future Opportunities
In order to overcome the various immunological barriers and improve clinical outcomes in cancer, immunotherapeutics must focus on stimulating different cellular immune pathways and overcoming the highly suppressive tumor microenvironment. IL-15-mediated immune cell activation by ALT-803 is expected to be attenuated by induction of immune checkpoint such as PD-1 expression by immune effector cells and PD-L1 expression by tumor cells23,24. Co-administration of checkpoint inhibitors, such as anti-PD-1 and anti-PD-L1, with ALT-803 may aid in overcoming the immunosuppressive tumor microenvironment and prolonging ALT-803-stimulated T and NK cell activity and persistence. Potent immune stimulation and blocking suppression can be achieved in a single therapeutic agent using the ALT-803 protein scaffold by combining the highly potent immunostimulatory cytokine, IL-15, with checkpoint inhibitors for preventing immune exhaustion, therapeutic monoclonal antibodies and TCRs for disease-cell targeting, and protein-based molecules for neutralizing immune suppression. Also, ALT-803 could be used to create a single molecule fused with various cytokines for exogenous expansion and stimulation of immune cells for adoptive cell therapy. Adding infectious agent-specific recognition domains to the ALT-803 scaffold has the potential to create novel fusion proteins against virally infected cells for treatment of infectious diseases as well. Thus, ALT-803 is versatile protein scaffold that provides an opportunity for creating unlimited numbers of novel and highly potent immunostimulatory fusion molecules for various clinical applications.
Conflict of interest statement: The authors are employees and shareholders of Altor BioScience.
References
- Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006; 6(8): 595-601.
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999; 17(7): 2105-2116.
- Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008; 222: 357-368.
- Rosenberg SA. Interleukin-2 and the development of immunotherapy for the treatment of patients with cancer. Cancer J Sci Am. 2000; 6 Suppl 1: S2-7.
- Waldmann T, Tagaya Y, Bamford R. Interleukin-2, interleukin-15, and their receptors. Int Rev Immunol. 1998; 16(3-4): 205-226.
- Waldmann TA, Lugli E, Roederer M, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011; 117(18): 4787-4795.
- Dubois S, Mariner J, Waldmann TA, et al. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002; 17(5): 537-547.
- Zhu X, Marcus WD, Xu W, et al. Novel human interleukin-15 agonists. J Immunol. 2009; 183(6): 3598-3607.
- Han KP, Zhu X, Liu B, et al. IL-15:IL-15 receptor alpha superagonist complex: high-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine. 2011; 56(3): 804-810.
- Rhode PR, Egan JO, Xu W, et al. Comparison of the Superagonist Complex, ALT-803, to IL15 as Cancer Immunotherapeutics in Animal Models. Cancer Immunol Res. 2016; 4(1): 49-60.
- Xu W, Jones M, Liu B, et al. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor alphaSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res. 2013; 73(10): 3075-3086.
- Basher F, Jeng EK, Wong H, et al. Cooperative therapeutic anti-tumor effect of IL-15 agonist ALT-803 and co-targeting soluble NKG2D ligand sMIC. Oncotarget. 2016; 7(1): 814-830.
- Kim PS, Kwilas AR, Xu W, et al. IL-15 superagonist/IL-15RalphaSushi-Fc fusion complex (IL-15SA/IL-15RalphaSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget. 2016; 7(13): 16130-16145.
- Mathios D, Park CK, Marcus WD, et al. Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model. Int J Cancer. 2016; 138(1): 187-194.
- Rosario M, Liu B, Kong L, et al. The IL-15-Based ALT-803 Complex Enhances FcgammaRIIIa-Triggered NK Cell Responses and In Vivo Clearance of B Cell Lymphomas. Clin Cancer Res. 2016; 22(3): 596-608.
- Allegrezza MJ, Rutkowski MR, Stephen TL, et al. IL15 Agonists Overcome the Immunosuppressive Effects of MEK Inhibitors. Cancer Res. 2016; 76(9): 2561-2572.
- Bailey CP, Budak-Alpdogan T, Sauter CT, et al. New interleukin-15 superagonist (IL-15SA) significantly enhances graft-versus-tumor activity. Oncotarget. 2017; 8(27): 44366-44378.
- Felices M, Chu S, Kodal B, et al. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol Oncol. 2017; 145(3): 453-461.
- Wagner JA, Rosario M, Romee R, et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J Clin Invest. 2017; 127(11): 4042-4058.
- Jochems C, Tritsch SR, Pellom ST, et al. Analyses of functions of an anti-PD-L1/TGFbetaR2 bispecific fusion protein (M7824). Oncotarget. 2017; 8(43): 75217-75231.
- Wong RL, Liu B, Zhu X, et al. Interleukin-15:Interleukin-15 receptor alpha scaffold for creation of multivalent targeted immune molecules. Protein Eng Des Sel. 2011; 24(4): 373-383.
- Liu B, Kong L, Han K, et al. A Novel Fusion of ALT-803 (Interleukin (IL)-15 Superagonist) with an Antibody Demonstrates Antigen-specific Antitumor Responses. J Biol Chem. 2016; 291(46): 23869-23881.
- Yu P, Steel JC, Zhang M, et al. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res. 2010; 16(24): 6019-6028.
- Yu P, Steel JC, Zhang M, et al. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci U S A. 2012; 109(16): 6187-6192.
