Anti-inflammatory Approaches to Mitigate the Neuroinflammatory Response to Brain-Dwelling Intracortical Microelectrodes
Hillary W. Bedell1,2 and Jeffrey R. Capadona1,2*
1Department of Biomedical Engineering, Case Western Reserve University, School of Engineering, 2071 MLK Jr. Drive, Wickenden Bldg, Cleveland OH 44106, USA
2Advanced Platform Technology Center, L. Stokes Cleveland VA Medical Center, Rehab. R&D, 10701 East Blvd. Mail Stop 151 AW/APT, Cleveland OH 44106, USA
Abstract
Intracortical microelectrodes are used both in basic research to increase our understanding of the nervous system and for rehabilitation purposes through brain-computer interfaces. Yet, challenges exist preventing the widespread clinical use of this technology. A prime challenge is with the neuroinflammatory response to intracortical microelectrodes. This mini-review details immunomodulatory strategies employed to decrease the inflammatory response to these devices. Over time, broad-spectrum anti-inflammatory approaches, such as dexamethasone and minocycline, evolved into more targeted treatments since the underlying biology of the neuroinflammation was elucidated. This review also presents studies which examine novel prospective targets for future immunomodulatory targeting.
Introduction: Brain-dwelling Intracortical Microelectrodes—Uses and Limitations
Intracortical microelectrodes were created as a tool for basic neuroscience research to understand the normal and diseased physiology of the brain for the treatment of neurological disorders, and they have since expanded to become critical components for brain-machine interfacing (BMI)1-3. BMI technologies have shown great success in enabling locked-in patients to interact with computers, robotic limbs, and their own electrically driven limbs3,4. The recent advances have inspired worldwide enthusiasm resulting in billions of dollars of federal and industrial sponsorships to promote understanding the brain for rehabilitative and preventative medicine applications. Additionally, private philanthropists have also demonstrated excitement in the field by investing in the use of brain interfacing technologies as a means to human augmentation5,6. In the future, brain-dwelling microelectrodes could restore patterns of normal brain function in diseased or injured patients through therapeutic modulation of dysfunctional pathways. Thus, implantable microelectrodes in the future can be transformative in not only restoring sensorimotor abilities but also improving the treatment of brain disorders and augmenting human capabilities.
While the promise of these incredible technologies is real, caution must be taken as implications regarding optimal performance and unforeseen side effects following device implantation into the brain are not fully characterized. For example, we recently demonstrated that rats with intracortical microelectrodes implanted in the primary motor cortex exhibited a remarkable 527% increase in the time required to complete a fine motor task7. Notably, the increased time to complete fine motor tasks was correlated with persistent damage to the blood-brain barrier, as a result of the neuroinflammatory response.
Despite the incredible enthusiasm for brain interfacing technologies, it is widely understood that microelectrodes for BMI technologies exhibit limited long-term viability where recordings typically fail 6 months to 1 year after implantation, due to multimodal failure mechanisms8. One of the primary causes for this failure is believed to originate from the perpetual inflammatory response following implantation9,10. Since the neurodegenerative progression of the disease and injured states are often associated with the progression of cognitive and motor-related disease symptoms, the relative contribution of implanting potentially restorative devices that could actually contribute to the perceived progression of disease-like symptoms remains unclear, and in most cases untested. Therefore, these critical questions need to be answered in respect to both understanding disease progression and the role of interfacing technologies in both treating and propagating disease-like symptoms of deteriorated cognitive and motor abilities.
Fortunately, much work is being done surrounding microelectrode design to reduce the inflammatory response (for review see8,11). Although significant progress has been made fabricating ultra-small implants, a universal approach to reduce inflammation regardless of the device decreases the need for design restrictions for successful tissue integration. For example, larger brain-dwelling electrodes are necessary to reach deep brain structures such as nucleus accumbens12. Additionally, in order to input/output more degrees of freedom from these neural prostheses, many electrode sites are needed resulting in designs with greater surface area13. Larger electrodes result in more foreign material in the brain disrupting tissue; the more invasive the implant, the more tissue is damaged during implantation. Ideally, lessons learned about the neuroinflammatory response to smaller intracortical microelectrodes can be applied to future applications of any brain-dwelling electrode, regardless of size or design. The factors that drive the inflammatory response must be targeted to reduce neuronal dysfunction and damage, to lead to improved signal for any application using implantable microelectrodes to ensure chronic, high-fidelity function.
An active immunomodulatory approach should afford the resolution of the damage caused during the implantation of the microelectrode, while also curtailing the chronic neurodegenerative aspects of the neuroinflammatory response to enhance the long-term function of the device. Furthermore, an immunomodulatory approach should also work in combination with innovative design strategies to combat the foreign body response, as no one solution can adapt as fast as the dynamic temporally facilitated biological response. In addition to modifications in the design of the probe to mitigate the inflammatory response, there are also biological and materials-based approaches undertaken to reduce neuroinflammation to chronically implanted intracortical microelectodes (reviewed in 8). In this mini-review, however, we focus on only direct immunomodulatory approaches applied to reduce the inflammatory response and improve the function of implantable intracortical microelectrodes, and we provide our current vision of the leading prospects moving forward.
Discussion
The following sections will discuss leading active anti-inflammatory approaches to mitigate microelectrode-induced neuroinflammation.
Steroids: Dexamethasone (DEX)
The first method to directly modulate the immune system after implantation of microelectrodes was the use of systemic injection of dexamethasone (DEX), commonly used for the treatment of multiple sclerosis in the clinic. DEX is a synthetic glucocorticoid that induces pleiotropic anti-inflammatory functions through cellular glucocorticoid receptors. Most cells, including microglia, express receptors for glucocorticoids such as dexamethasone14.
The first group to report the use of DEX to minimize neuroinflammation in response to intracortical microelectrodes peripherally injected DEX (200 mg/kg) in rats daily for six days beginning the day of surgery. The systemic administration of DEX led to decreased astrocytic response at 1 and 6 weeks post-implantation15. However, the same systemic repeated administration of DEX at a lower dose (200 µg/kg) in rats, led to a “transient increase” in both microglial/macrophage responses and laminin deposition which was interpreted by the authors as a response to injury, repair, and/or angiogenesis16. Since high systemic delivery of steroids can have serious side effects, DEX has also been incorporated into different probe coatings: poly(ethyl-vinyl) acetate, nitrocellulose, carbon nanotubes, and poly(lactic-co-glycolic acid) nanoparticles within alginate hydrogel matrices17,18. Each of the studies delivering DEX locally examined different markers of neuroinflammation. Benefits common to all the studies included decreased astrocytic response, reduced microglial/macrophage activity, mitigated neuronal loss, and minimized chondroitin sulfate proteoglycan expression15,19-21.
DEX has also been shown to improve the functional recording performance of intracortical microelectrode. For example, DEX was loaded into electrospun biodegradable nano-fibers followed by an alginate hydrogel encapsulation to allow for long-term release. Poly (3,4-ethylene dioxythiophene) (PEDOT) was then electrochemically polymerized on the electrode sites within the scaffold. The composite coating with PEDOT elicited decreased impedance compared to non-PEDOT coated electrodes and allowed for higher applied charge density than traditional electrodes22. DEX incorporated into nanoparticle embedded coatings reduced impedance compared to no-drug controls by 25%, likely due to the demonstrated decreased tissue response23. Zhong and colleagues were able to release DEX from nitrocellulose coatings for 16 days in vitro. However, once the drug is depleted from the probe material, it is currently not possible to reload the material with the drug, Furthermore, acute strategies to mitigate the inflammatory response have not been completely successful at more chronic time points24,25. Currently, continuous local delivery of a drug such as DEX can be delivered in vitro on a 1.5 mm metal neural probe using microfluidic technology26. As the development of this technology progresses to incorporate a microfluidic delivery system into smaller, functional, neural probes, local continuous delivery of DEX can be a promising strategy in a larger multifaceted anti-inflammatory approach.
Antibiotics
Another broad-spectrum drug that has been investigated to decrease neuroinflammation associated with microelectrode implantation is minocycline. Minocycline is a semi-synthetic tetracycline shown to be neuroprotective in brain and spinal cord injury27. Rennekar and colleagues were the first group to use minocycline to improve neural recordings from intracortical microelectrodes. The team dissolved minocycline-HCl in the drinking water of experimental rats for 2 days prior to probe implantation through 5 days post-implantation. It was estimated that the rats received about 4 mg minocycline per day (13–20 mg kg−1), based on water consumption. After 6 days, the group receiving minocycline yielded significantly higher signal to noise ratios (SNR) and the percentage of active channels detecting neural activity was higher than the control group. Both metrics for improved recording quality were maintained through 4 weeks post-implantation (study completion). Additionally, glial scarring was reduced with minocycline administration at both of the time points in which histology was examined, 1 and 4 weeks post-implantation28. Unfortunately, no report of neuronal density, known to die back around the implants, was provided in Rennekar’s study.
An earlier study had previously shown that to achieve neuroprotective effects, minocycline must be present by the neurons at much higher levels29,30. Thus, Zhang et al. incorporated minocycline into thin film coatings on oxidized silicon, a common material for neural electrodes31. The thin film coatings allow for increased loading and local, sustained release of higher concentrations of minocycline (over 46 days). In preliminary in vitro testing, minocycline incorporated in thin film coatings were able to elicit neuroprotective activity similar to controls dispersed directly into the culture media. Although these studies were conducted in vitro, the results demonstrated promise for the extended delivery of minocycline via neural probes. Additionally, recent work showed that minocycline delivered through microfluidic channels fabricated within an implanted neural probe led to reductions in microglial reactions between 200-400 µm from the probe interface32. Such distances are unlikely to impact neural recording quality. Interestingly, minocycline delivery had no effect on astrocyte density, inconsistent with the original findings from Rennekar et al. Similarly, in concordance to the previously mentioned studies, Hayn et al. (2017) administered minocycline in a single local dose (20 µg/µL) via cannula implantation in the motor cortex of rats and found decreased neuronal death and anti-inflammatory effects with improved motor function33. Overall, these studies demonstrate promise for minocycline to mitigate the neuroinflammatory response around implanted microelectrodes.
However, long-term dosing of minocycline begets an increased risk of adverse events—including hyperpigmentation of the skin and other organs34,35. Minocycline possesses an increased chance of serious adverse events relative to other tetracyclines35. Thus, minocycline could be part of a multi-faceted approach to reduce initial neuroinflammation, but less risky alternative therapies are needed for chronic applications.
Because of an increased understanding of the biological response to microelectrode implantation, more specific immunomodulatory approaches are being utilized to mitigate the inflammatory response and improve the chronic performance of these devices.
Mitigating the Glial Response
Both microglia and astrocytes contribute to the biological response affecting electrode function. Thus, an effective way to modulate the immune response would be to mitigate the glial response. The Bellamkonda group utilized alpha-melanocyte stimulating hormone (MSH) (an endogoneous tridecapetide) to target the microglial response36. Alpha-MSH has been shown to inhibit both nitric oxide and pro-inflammatory cytokines produced by activated microglia—both of which are detrimental to neuronal health37. In their study, the Bellamkonda group coupled the Alpha-MSH peptide to silicon single shank planar microprobes and implanted the probes into the motor cortex of rats. The astrocytic and activated microglial response was examined via histology at 1 and 4 weeks post-implantation. At both time points, the activated microglial response was significantly decreased compared to a non-coated control probe. There was no notable difference in astroglial scarring at 1 week, but at 4 weeks post-implantation significantly less scarring was present36.
Purcell et al. administered flavopiridol to rats implanted with Michigan-style single shank silicon multi-channel intracortical electrodes38. Flavopiridol arrests progression into the cell cycle, yet, re-entry into the cell cycle has been demonstrated in glial activation as shown by upregulation of cell-cycle components39. Thus, flavopiridol was hypothesized to lead to decreased glial activation and improved intracortical microelectrode recording performance. However, Purcell et al. were unable to demonstrate improved neural recording or decreased presence of glial cells (astrocytes and microglia combined)38.
Another immunomodulatory approach that has been explored was to use a cytokine receptor antagonist to reduce neuroinflammation. Interleukin- 1 receptor antagonist (IL-1Ra) has been used in human clinical trials for rheumatoid arthritis treatment and is endogenously released by microglia to facilitate neuroprotection40,41. Neural probe coatings incorporated with IL-1Ra have been successfully used to improve neuronal survival and reduce glial scarring as long as four weeks post-implantation42,43. Unfortunately, no information has been reported to date about either chronic time points or the effects on recording performance.
Targeting the Inflammasome
Given the role of the inflammasome in brain injury models, targeting the players of this construct can be pivotal in reducing neuroinflammation following intracortical microelectrode implantation. The inflammasome is an innate immune protein complex. The inflammasome activates caspase-1, an enzyme which allows a pivotal pro-inflammatory cytokine, IL- 1β, to achieve its active form. IL-1β has been shown to be significantly upregulated around intracortical probes and plays a role in blood-brain barrier (BBB) dysfunction11,44. Kozai et al. used a knock-out mouse model to demonstrate caspase-1 as a promising immunomodulatory target for improving chronic single-unit recordings by intracortical microelectrodes implanted in the visual cortex of mice45. Data obtained by Kozai et al. suggested that pharmacologic interventions which target the components and downstream players of the inflammasome can yield more stable chronic neural recordings. Future studies could entail using a caspase inhibitor such as VX-765 or Ac-YVAD-cmk, both of which have demonstrated neuroprotection in various models46-48.
Targeting Blood-derived Cells
Blood-derived cells have been shown to be major contributors to the inflammatory response of brain-dwelling intracortical microelectrode, despite the perception of the immune privileged brain49,50. Thus, a recently developed hypothesis is that immunomodulatory strategies that limit blood-derived immune cells from the area within the brain, surrounding the implanted neural electrodes, could mitigate the inflammatory response and concurrently improve recording performance. Targeting monocyte chemoattractant protein-1 (MCP-1) using a knock out mouse model was found to decrease the inflammatory response to intracortical microelectrode implantation51. MCP-1 is a chemoattractant which attracts monocytes to areas of inflammation, such as the response to a neural electrode in the brain. Although this study did not directly evaluate reductions to blood-derived cells, they found that MCP-1 knock-out mice reduced BBB leakage, microglia/macrophage response, and astrocytic response, all leading to an increased neuronal density at acute and chronic time points. Notably, the improved neuroinflammatory response was recapitulated with pharmacological inhibition of MCP-1 via daily intraperitoneal injections51. It will be critical for future studies to also investigate the impact on neural recordings.
Pattern Recognition Receptors
Given the presence of blood and cellular damage present at the tissue-device interface after implantation, targeting cellular receptors that recognize damage and initialize the production of pro-inflammatory cytokines promises to reduce the inflammatory response and improve intracortical microelectrode performance. Pattern recognition receptors detect cellular damage and blood proteins and are found on microglia, neurons, astrocytes, and blood-derived macrophages present at the probe interface. Through the use of knock-out mouse models and a small-molecule inhibitor, we have previously shown that targeting the toll-like receptor (TLR)/cluster of differentiation 14 (CD14) pathways can improve both acute and chronic microelectrode performance49,52. Thus, pattern recognition receptors are amenable targets to improve neural recording from brain-dwelling intracortical microelectrodes, yet appropriate dosing regimens for optimal wound healing and anti-inflammatory responses remains to be determined.
Conclusion and Perspectives
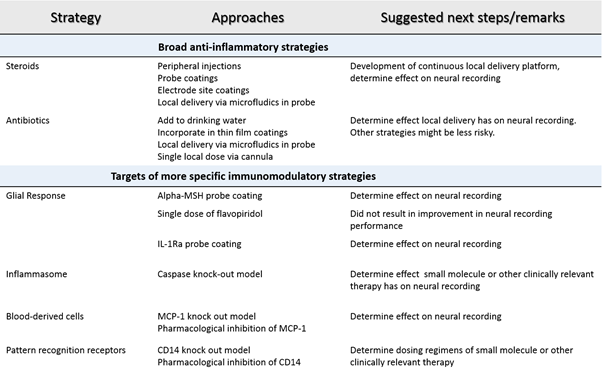
The goal of our mini-review was to highlight key studies in the many parallel approaches to mitigating the neuroinflammatory response to brain-dwelling neural electrodes (Table 1). The mini-review was intended as a starting point for discussion towards the integration of a more targeted approach to device integration. As the list of interesting targets within the brain for interfacing continues to grow, a more comprehensive and specific strategy for device integration will be required. Electrode design alone will not be the answer. As a more detailed understanding of the biology at the probe-tissue interface is elucidated, more directed therapeutic interventions which modulate the immune system will be required to improve device performance to a long-term, reliable system. It should be clear that many promising approaches to mitigation of inflammation are under development and studies looking at the effects of neural recording to many of these approaches need to be conducted before the aforementioned approaches are widely adapted. Strategies that utilize immunomodulatory interventions already in the clinic will need to be coupled with responsive local delivery platforms to avoid peripheral anti-inflammatory treatment that should not be a long-term solution. Large animal and clinical demonstration of such approaches will soften the barrier to novel immunomodulatory strategies at earlier stages of development and ultimately bring forth significant benefit to the function of these devices and the rehabilitation of disabled individuals.
Table 1: Summary of strategies to mitigate the neuroinflammatory response to intracortical microelectrodes.
Funding Sources
Support for the authors was provided in part by the National Institute of Health, National Institute of Neurological Disorders and Stroke, (Grant # 1R01NS082404-01A1), the Office of the Assistant Secretary of Defense for Health Affairs through the Peer Reviewed Medical Research Program (Award No. W81XWH-15-1-0608), the Presidential Early Career Award for Scientists and Engineers (PECASE), and by Merit Review Award (B1495-R and B2611-R) from the United States (US) Department of Veterans Affairs Rehabilitation Research and Development Service. None of the funding sources aided in collection, analysis and interpretation of the data, in writing of the manuscript, or in the decision to submit the manuscript for publication. The authors have no conflict of interest related to this work to disclose. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
References
- Golub MD, Chase SM, Batista AP, et al. Brain–computer interfaces for dissecting cognitive processes underlying sensorimotor control. Current opinion in neurobiology. 2016; 37: 53-58.
- Collinger JL, Wodlinger B, Downey JE, et al. High-performance neuroprosthetic control by an individual with tetraplegia. The Lancet. 2013; 381(9866): 557-564.
- Ajiboye AB, Willett FR, Young DR, et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. The Lancet. 2017; 389(10081): 1821-1830.
- Donoghue J. Bridging the Brain to the World: A Perspective on Neural Interface Systems. Neuron. 2008; 60(3): 511-521.
- Winkler R. Elon Musk Launches Neuralink to Connect Brains With Computers. The Wallstreet Journal. 2017.
- Wu J, Rao RPN. How close are we to Elon Musk's brain-computer interface? 2017; http://www.cnn.com/2017/04/12/health/brain-computer-interface-partner/.
- Goss-Varley M, Dona KR, McMahon JA, et al. Microelectrode implantation in motor cortex causes fine motor deficit: Implications on potential considerations to Brain Computer Interfacing and Human Augmentation. Scientific Reports. 2017; 7: 15254.
- Jorfi M, Skousen JL, Weder C, et al. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J Neural Eng. 2015; 12(1): 011001.
- Nolta NF, Christensen MB, Crane PD, et al. BBB leakage, astrogliosis, and tissue loss correlate with silicon microelectrode array recording performance. Biomaterials. 2015; 53: 753-762.
- Barrese JC, Rao N, Paroo K, et al. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng. 2013; 10(6): 066014.
- Karumbaiah L, Saxena T, Carlson D, et al. Relationship between intracortical electrode design and chronic recording function. Biomaterials. 2013; 34(33): 8061-8074.
- Wu H, Miller KJ, Blumenfeld Z, et al. Closing the loop on impulsivity via nucleus accumbens delta-band activity in mice and man. Proceedings of the National Academy of Sciences. 2018; 115(1): 192-197.
- Lebedev MA, Nicolelis MA. Brain–machine interfaces: past, present and future. TRENDS in Neurosciences. 2006; 29(9): 536-546.
- Reichardt HM, Tuckermann JP, Göttlicher M, et al. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. The EMBO journal. 2001; 20(24): 7168-7173.
- Shain W, Spataro L, Dilgen J, et al. Controlling cellular reactive responses around neural prosthetic devices using peripheral and local intervention strategies. IEEE transactions on neural systems and rehabilitation engineering. 2003; 11(2): 186-188.
- Spataro L, Dilgen J, Retterer S, et al. Dexamethasone treatment reduces astroglia responses to inserted neuroprosthetic devices in rat neocortex. Experimental neurology. 2005; 194(2): 289-300.
- Hempen C, Weiss E, Hess CF. Dexamethasone treatment in patients with brain metastases and primary brain tumors: do the benefits outweigh the side-effects. Supportive care in cancer. 2002; 10(4): 322-328.
- Heimdal K, Hirschberg H, Slettebø H, et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. Journal of neuro-oncology. 1992; 12(2): 141-144.
- Kim DH, Martin DC. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006; 27(15): 3031-3037.
- Zhong Y, Bellamkonda RV. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain research. 2007; 1148: 15-27.
- Luo X, Matranga C, Tan S, et al. Carbon nanotube nanoreservior for controlled release of anti-inflammatory dexamethasone. Biomaterials. 2011; 32(26): 6316-6323.
- Abidian MR, Martin DC. Multifunctional Nanobiomaterials for Neural Interfaces. Advanced Functional Materials. 2009; 19(4): 573-585.
- Mercanzini A, Reddy ST, Velluto D, et al. Controlled release nanoparticle-embedded coatings reduce the tissue reaction to neuroprostheses. Journal of Controlled Release. 2010; 145(3): 196-202.
- Potter KA, Jorfi M, Householder KT, et al. Curcumin-releasing mechanically adaptive intracortical implants improve the proximal neuronal density and blood–brain barrier stability. Acta biomaterialia. 2014; 10(5): 2209-2222.
- Szarowski D, Andersen M, Retterer S, et al. Brain responses to micro-machined silicon devices. Brain research. 2003; 983(1-2): 23-35.
- Frey L, Bandaru P, Zhang YS, et al. A Dual?Layered Microfluidic System for Long?Term Controlled In Situ Delivery of Multiple Anti?Inflammatory Factors for Chronic Neural Applications. Advanced Functional Materials. 2018; 28(12): 1702009.
- Yong VW, Wells J, Giuliani F, et al. The promise of minocycline in neurology. The Lancet Neurology. 2004; 3(12): 744-751.
- Rennaker R, Miller J, Tang H, et al. Minocycline increases quality and longevity of chronic neural recordings. Journal of neural engineering. 2007; 4(2): L1.
- Pi R, Li W, Lee NT, et al. Minocycline prevents glutamate?induced apoptosis of cerebellar granule neurons by differential regulation of p38 and Akt pathways. Journal of neurochemistry. 2004; 91(5):1219-1230.
- Garcia-Martinez EM, Sanz-Blasco S, Karachitos A, et al. Mitochondria and calcium flux as targets of neuroprotection caused by minocycline in cerebellar granule cells. Biochemical pharmacology. 2010; 79(2): 239-250.
- Zhang Z, Nong J, Zhong Y. Antibacterial, anti-inflammatory and neuroprotective layer-by-layer coatings for neural implants. Journal of neural engineering. 2015; 12(4): 046015.
- Liu B, Kim E, Meggo A, et al. Enhanced biocompatibility of neural probes by integrating microstructures and delivering anti-inflammatory agents via microfluidic channels. Journal of neural engineering. 2017; 14(2): 026008.
- Hayn L, Deppermann L, Koch M. Reduction of the foreign body response and neuroprotection by apyrase and minocycline in chronic cannula implantation in the rat brain. Clinical and Experimental Pharmacology and Physiology. 2017; 44(2): 313-323.
- Reed DN, Gregg FO, Corpe RS. Minocycline-induced black bone disease encountered during total knee arthroplasty. Orthopedics. 2012; 35(5): e737-e739.
- Ochsendorf F. Minocycline in acne vulgaris. American journal of clinical dermatology. 2010; 11(5): 327-341.
- He W, McConnell GC, Schneider TM, et al. A novel anti?inflammatory surface for neural electrodes. Advanced Materials. 2007; 19(21): 3529-3533.
- Galimberti D, Baron P, Meda L, et al. α-MSH peptides inhibit production of nitric oxide and tumor necrosis factor-α by microglial cells activated with β-amyloid and interferon γ. Biochemical and biophysical research communications. 1999; 263(1): 251-256.
- Purcell EK, Thompson DE, Ludwig KA, et al. Flavopiridol reduces the impedance of neural prostheses in vivo without affecting recording quality. Journal of neuroscience methods. 2009; 183(2): 149-157.
- Di Giovanni S, Movsesyan V, Ahmed F, et al. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proceedings of the National Academy of Sciences of the United States of America. 2005; 102(23): 8333-8338.
- Pinteaux E, Rothwell NJ, Boutin H. Neuroprotective actions of endogenous interleukin?1 receptor antagonist (IL?1ra) are mediated by glia. Glia. 2006; 53(5): 551-556.
- Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine & growth factor reviews. 2002; 13(4-5): 323-340.
- Gutowski SM, Shoemaker JT, Templeman KL, et al. Protease-degradable PEG-maleimide coating with on-demand release of IL-1Ra to improve tissue response to neural electrodes. Biomaterials. 2015; 44: 55-70.
- Taub AH, Hogri R, Magal A, et al. Bioactive anti?inflammatory coating for chronic neural electrodes. Journal of biomedical materials research Part A. 2012; 100(7): 1854-1858.
- Tian W, Kyriakides TR. Matrix metalloproteinase-9 deficiency leads to prolonged foreign body response in the brain associated with increased IL-1β levels and leakage of the blood-brain barrier. Matrix Biology. 2009; 28(3): 148-159.
- Kozai TD, Li X, Bodily LM, et al. Effects of caspase-1 knockout on chronic neural recording quality and longevity: Insight into cellular and molecular mechanisms of the reactive tissue response. Biomaterials. 2014; 35(36): 9620-9634.
- Wannamaker W, Davies R, Namchuk M, et al. (S)-1-((S)-2-{[1-(4-Amino-3-chloro-phenyl)-methanoyl]-amino}-3, 3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2R, 3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an Orally Available Selective Interleukin (IL)-Converting Enzyme/Caspase-1 Inhibitor, Exhibits Potent Anti-Inflammatory Activities by Inhibiting the Release of IL-1β and IL-18. Journal of Pharmacology and Experimental Therapeutics. 2007; 321(2): 509-516.
- Rabuffetti M, Sciorati C, Tarozzo G, et al. Inhibition of caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone induces long-lasting neuroprotection in cerebral ischemia through apoptosis reduction and decrease of proinflammatory cytokines. Journal of Neuroscience. 2000; 20(12): 4398-4404.
- Bassil F, Fernagut PO, Bezard E, et al. Reducing C-terminal truncation mitigates synucleinopathy and neurodegeneration in a transgenic model of multiple system atrophy. Proceedings of the National Academy of Sciences. 2016; 113(34): 9593-9598.
- Bedell HW, Hermann JK, Ravikumar M, et al. Targeting CD14 on blood derived cells improves intracortical microelectrode performance. Biomaterials. 2018; 163: 163-173.
- Ravikumar M, Sunil S, Black J, et al. The Roles of Blood-derived Macrophages and Resident Microglia in the Neuroinflammatory Response to Implanted Intracortical Microelectrodes. Biomaterials. 2014; S0142-9612(35): 8049-8064.
- Sawyer AJ, Tian W, Saucier-Sawyer JK, et al. The effect of inflammatory cell-derived MCP-1 loss on neuronal survival during chronic neuroinflammation. Biomaterials. 2014; 35(25): 6698-6706.
- Hermann JK, Ravikumar M, Shoffstall AJ, et al. Inhibition of the cluster of differentiation 14 innate immunity pathway with IAXO-101 improves chronic microelectrode performance. Journal of Neural Engineering. 2018; 15(2): 025002.
