Controlling the cGAS-STING Pathway: The Signature of ASFV Virulence
Raquel García-Belmonte, Daniel Pérez-Núñez, Yolanda Revilla*
Centro de Biología Molecular Severo Ochoa, CSIC-UAM, Microbes in Health and Welfare Department, c/ Nicolás Cabrera, 1, 28049 Madrid, Spain
African swine fever virus (ASFV) is a dsDNA virus that infects domestic pigs and wild boar, which is currently expanding throughout the world representing a huge concern for pig industry. Highly virulent strains cause high mortality in domestic pigs, while attenuated strains induce chronic illness. We have described that virulence of ASFV strains depends on their ability to control innate immune response through the IFN-I production through the GAS-STING pathway control. Sensing of cytosolic dsDNA by cGAS following of cGAMP production, triggers further signaling through STING, TBK1, and IRF3, leading to the production of type I IFNs. While ASFV attenuated NH/P68 activated the cGAS-STING pathway very early during infection and consequently IFN-β was produced, Armenia/07 infection provoked the inhibition of this pathway blocking the production of IFN-β. These findings showed for the first time the involvement of the cGAS-STING-IRF3 route in ASFV infection and its involvement in virulence determination. Since then, several ASFV proteins that control this pathway have been described. These genes target several components of the pathway such as STING, TBK1 or IRF3, highlighting the importance of this route for the virulent infection. In this minireview we shortly summarize the current knowledge on the molecular mechanisms leading by ASFV to control cGAS-STING pathway.
African Swine Fever Virus
African swine fever virus (ASFV) is a complex cytoplasmic dsDNA virus, which targets monocytes-macrophages of domestic pigs and wild boars causing an acute deadly hemorrhagic viral disease, African swine fever (ASF). ASF causes important economic consequences to the pig industry and to the ecosystem in affected places and currently is spreading through Asia, Europe and Oceania affecting more than 30 countries and very recently an outbreak has been reported in Dominican Republic 1. ASF manifestations include subacute, acute as well as chronical forms 2 depending on the virulence of the viral strain. Highly virulent strains are responsible for subacute and acute ASF characterized by causing death in a few days. Chronic ASF is normally caused by attenuated strains and is not lethal. These differences in virulence among different ASFV strains suggests a distinct activation of the immune system through the regulation of virus-host interaction 3–5.
cGAS-STING Pathway Control During ASFV Infection
Innate immune system is the first line of host defense during infection. Pathogen associated molecular patterns (PAMPs) are recognized by pattern recognition receptors (PRRs) activating signaling pathways to induce innate immune response, leading to the production of type I interferon (IFN-I), pro-inflammatory cytokines and chemokines. In mammals, cytosolic detection of nucleic acids is critical in initiating innate antiviral responses, which are mediated by multiple cytosolic and endosomal sensors and adaptor molecules, including the cGAS-STING pathway. cGAS (cyclic GMP-AMP (cGAMP) synthase) protein is a PPR that acts as cytosolic DNA sensor, which undergoes conformational rearrangement when binding DNA producing cGAMP, which in turn binds and activates the Stimulator of Interferon Genes protein (STING), inducing downstream signaling leading to IFN-I production 6.cGAMP binding induces STING dimerization and allows STING trafficking from endoplasmic reticulum (ER) to the trans-Golgi network (TGN) where tank binding kinase 1 (TBK1) is recruited to phosphorylate STING in Ser366, within its pLxIS motif. This phosphorylation is necessary for the interaction between Interferon regulatory factor 3 (IRF3) and STING, which occurs through the pLxIS motif and allows IRF3 phosphorylation in Ser396 by TBK1 7. Finally, IRF3 dimerizes and translocate to the nucleus where acts as a transcription factor, inducing IFN-b production 8.
In García-Belmonte et al. 9, we describe for the first time the virulence associated with different ASFV strains with their ability to regulate IFN-I production through the control of cGAS-STING pathway. We described how the circulating virulent Armenia/07 strain, and not the attenuated NH/P68, inhibits cGAS-STING pathway to counteract IFN-b production in porcine alveolar macrophages (PAM). Interestingly, at early times post infection, both virulent and attenuated strains triggered cGAS-STING activation, but only the virulent strain controls critical steps to inhibit IFN-b production. We identified cGAS as the main DNA sensor involved in STING activation upon ASFV infection, since a specific inhibitor of cGAS, Ru521 10, blocked the activation of STING produced by the infection.
After cGAS-STING pathway activation, STING trafficking from ER to the TGN is needed for the recruitment and activation of TBK1, a key component of the pathway. Upon activation, STING translocates from the ER to the Golgi compartment, and then, to perinuclear punctuated structures, called microsomes 11–13. These microsomes appeared in most of the NH/P68-infected cells at 6 hours post infection (hpi), whereas during Armenia/07 infection STING was mainly found dispersed throughout the cytoplasm, indicating the blockade of STING trafficking by the virulent strain.
After STING translocation to the Golgi compartments, phosphorylation of STING, TBK1 and IRF3, and the subsequent translocation of p-IRF3 to the nucleus are the following events leading to IFN-I production. STING and IRF3 showed a distinct pattern of phosphorylation during NH/P68 or Armenia/07 depending on the different post-infection times. While NH/P68 induced high levels of STING and IRF3 phosphorylation, Armenia/07 induced a slight phosphorylation of both proteins as early as 2 hpi, which was abolished at later times, demonstrating the Armenia/07 ability to inhibit STING and IRF3 phosphorylation, according to previous results.
The involvement of the cGAS-STING pathway in the control of IFN-b production during ASFV infection was not previously described. Our work establishes the differences between attenuated and virulent strains regarding their ability to control the innate immune response. Moreover, we showed the blockage of STING phosphorylation by Armenia/07 in presence of the DNA replication inhibitor AraC, indicating this events happens before viral DNA replication 9, thus suggesting the possible candidate(s) should be early and immediately early ASFV genes 14.
ASFV Proteins Counteracting STING
After our publication on ASFV control of cGAS-STING pathway, several researchers have tried to characterize the ASFV genes responsible of ASFV escape from host antiviral innate response, and specifically the production of IFN-b induced by cGAS-STING pathway (see Figure 1). Li et al., 15 recently reported MGF505-7R as a candidate to modulate cGAS-STING pathway. They identified MGF505-7R markedly inhibiting cGAS-STING–mediated IFN-b and ISRE activation by targeting STING and STING upstream components, but not other downstream components as TBK1. They also found MGF505-7R interaction with STING but not with TBK1, cGAS or IRF3 in ectopic experiments and described STING degradation during either the ectopic expression of MGF505-7R or during ASFV wild-type virulent infection. The induction of the autophagy-related protein ULK1 during the expression of the MGF505-7R was suggested to be responsible for STING degradation, which it´s likely to be a controversial hypothesis as ULK1 was in fact inhibited in PAM infected with MGF505-7R deletion mutant 15. Degradation of STING was not found in that work 9, where downregulation of the STING phosphorylation was described, whereas the total levels of STING were conserved. Yang et al. 16, described other member of MGF505 family, MGF505-11R, to be able to bind STING inducing IFN-β modulation. Ectopic expression of MGF505-11R inhibits IFN-β and ISRE activation, binds STING and promotes STING degradation by the lysosome, ubiquitin-proteasome, and autophagy pathways 16. Unfortunately, Yang et al., did not confirm these results in infection experiments. For that reason, even though both genes could be involved in control of the IFN-b production, the data provided in Li et al., give an added value due to the experiments carried out with the deletion virus compared to wild type virus.
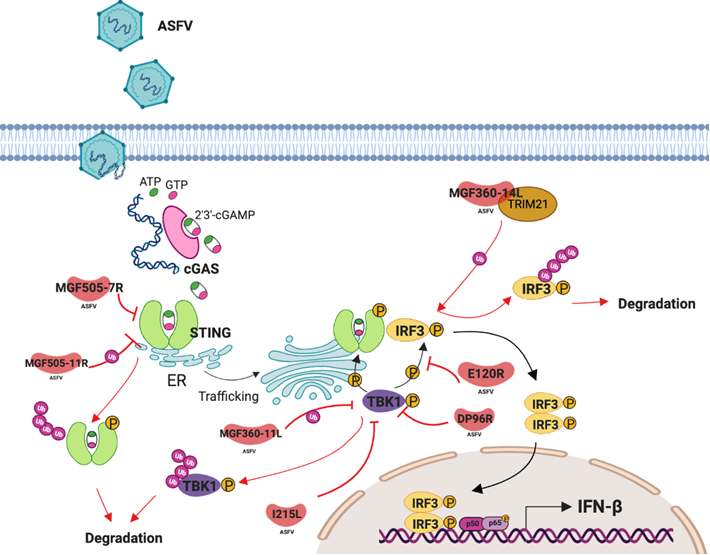
Figure 1: Reported genes to be involved in ASFV modulation of cGAS-STING pathway.
ASFV Proteins Counteracting TBK1
Not only STING has been directly described to interact and be modulated by ASFV proteins, also TBK1 has been reported. Wang et al., postulated that DP96R could have a role in the control of the cGAS-STING pathway by interaction of DP96R with TBK1. According to these authors, the C-terminal domain of DP96R interacts with TBK1 and inhibits its phosphorylation and in consequence IFN-β production 17. MGF360-11L has been also described to interact with TBK1 and IRF7 to downregulate the IFN-b production, by inducing TBK1 and IRF3 degradation 18. Nevertheless we did not observe modulation of total levels neither of STING, nor TBK1 and IRF3, but downregulation of phosphorylation levels of these factors 9. Other important postranslational modification, apart from phosphorylation is ubiquitination. Depending on which lysine is ubiquitinated, the effect could promote TBK1-degradation, or induce TBK1-activation, such as K63-linked polyubiquitination19. Huang et al.20 showed I215L as one of the strongest inhibitors in modulating IFN-I, by inhibiting K63-linked polyubiquitination. According to these authors, I215L recruted ubiquitin E3 ligase RNF138, enhancing RNF138 degradation, which is responsible to mediate K63 linked ubiquitination of TBK1 20.
ASFV Proteins Counteracting IRF3
IRF3 is the main transcription factor that binds to IFN-b promoter and allows the IFN-b production. Importantly, IRF3 is not only activated by cGAS-STING pathway, but it is also modulated by other cell signaling pathways as RIG-I. Anyhow, in all cases, the activation of IRF3 needs phosphorylation and translocation to the nucleous. ASFV proteins as MGF360-14L or E120R have been recently described to modulate IFN-b by targeting IRF3. Wang et al. 21 reported the degradation of IRF3 during ectopic expression of ASFV MGF360-14L. Authors proposed that MGF360-14L interacts with E3 ligase TRIM21, that mediates K63-linked ubiquitination of IRF3 allowing its degradation. However, Liu. et al, described IRF3 modulation as regulated by E120R interaction, a late viral protein of ASFV, specifically through the 72 and 73 aminoacid of E120R22. They explained that this interaction impairs the interaction between IRF3 and TBK1 resulting in no phosphorylation of IRF3 and hence no IFN-β production. However, the fact of E120R is a late protein in the viral cycle makes this afirmation controversial. However, interestingly, the authors performed a recombinant virus mutating these aminoacids in E120R and this modification was apparently enough to induce higher levels of IFN-b during infection, compared to ASFV wild type infection 22.
Table 1: Viral ASFV genes/proteins involved in cGAS-STING modulation.
|
Gene |
Early or late expression |
Cellular target |
Mechanism |
Model |
References |
|
MGF505_7R |
Early |
STING |
ULK1 induction to degrade STING |
Ectopic expression: HEK/293T, Ma_104, HeLa, PK15 Infection: PAM and in vivo assay #ASFVDMGF505_7R |
15 |
|
MGF505_11R |
Early |
STING |
STING degradation |
Ectopic expression: HEK/293T, PK15
|
16 |
|
MGF460_11L |
Ambivalent |
TBK1 |
TBK1 degradation |
Ectopic expression: HEK/293T, 3D4/21, PK15
|
18 |
|
I215L |
Early |
TBK1 |
Recruits RFN138 and induces K63-Ub to TBK1 |
Ectopic expression: HEK/293T
|
20 |
|
DP96R |
Early |
TBK1 |
Inhibits TBK1 phosphorylation |
Ectopic expression: HEK/293T, BHK_21
|
17 |
|
E120R |
Late |
IRF3 |
Impairs TBK1-IRF3 interaction |
Ectopic expression: HEK/293T, BHK_21, Ma_104 Infection: PAM and BMDM #E120R-D72-73aa |
22 |
|
MGF360_14L |
Early |
IRF3 |
Interacts with TRIM21 to induce K63-Ub to IRF3 |
Ectopic expression: HEK/293T, PK15
|
21 |
All these innovations highlight the importance of controlling the production of IFN-β during infection with ASFV, and in particular cGAS-STING pathway (Table 1). The efficacity over this control would determine the virulence of the strain in vivo. For this reason, ASFV needs to modulate the cGAS-STING pathway through apparently several genes to ensure the progression of the infection in the animal.
Perspectives
ASFV proteins counteracting the cGAS-STING pathway constitute valuable tools for the understanding of ASFV control on IFN-I, which is of key importance in virus-host interaction and will help to reveal the molecular mechanims of ASFV virulence. New high-throughput screening could be performed in order to identify new potential viral proteins involved on cGAS-STING pathway regulation interference. Animal studies that brings together all variables during ASFV infection and interaction among cellular and viral different actors will be of great help. In this regard, the emergence of new single cell-based technologies, such as single-cell RNA seq or CITE-seq, are promising tools to be employed in this exciting field of study.
References
- Gonzales W, Moreno C, Duran U, et al. African swine fever in the Dominican Republic. Transbound Emerg Dis. 2021; 68(6): 3018-3019. doi:10.1111/tbed.14341
- Gómez-Villamandos JC, Bautista MJ, Sánchez-Cordón PJ, et al. Pathology of African swine fever: The role of monocyte-macrophage. Virus Res. 2013; 173(1): 140-149. doi:10.1016/j.virusres.2013.01.017
- Pan IC HW. Virulence in African swine fever: its measurement and implications. Am J Vet Res. 1984; 45: 361–366.
- Pini A WG. Isolation of a non-haemadsorbing strain of African swine fever (ASF) virus from a natural outbreak of the disease. Vet Res. 1974; 94(2). doi:https://doi.org/10.1136/vr.94.1.2.
- Vigario JD, Terrinha AM MNJ. Antigenic relationships among strains of African swine fecre virus. Arch Gesamte Virusforsch. 1974; 45: 272-277. doi:https://doi.org/10.1007/BF01249690
- Barber GN. STING-dependent cytosolic DNA sensing pathways. Trends Immunol. 2014; 35(2): 88-93. doi:10.1016/j.it.2013.10.010
- Liu S, Cai X, Wu J, et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science (80- ). 2015; 347(6227). doi:10.1126/science.aaa2630
- Ablasser A, Chen ZJ. CGAS in action: Expanding roles in immunity and inflammation. Science (80- ). 2019; 363(6431). doi:10.1126/science.aat8657
- García-Belmonte R, Pérez-Núñez D, Pittau M, et al. African Swine Fever Virus Armenia/07 Virulent Strain Controls Interferon Beta Production through the cGAS-STING Pathway. J Virol. 2019; 93(12): 1-17. doi:10.1128/jvi.02298-18
- Vincent J, Adura C, Gao P, et al. Small molecule inhibition of cGAS reduces interferon expression in primary macrophages from autoimmune mice. Nat Commun. 2017; 8(1): 1-12. doi:10.1038/s41467-017-00833-9
- Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type i interferon-dependent innate immunity. Nature. 2009; 461(7265): 788-792. doi:10.1038/nature08476
- Mukai K, Konno H, Akiba T, et al. Activation of STING requires palmitoylation at the Golgi. Nat Commun. 2016; 7(May): 1-10. doi:10.1038/ncomms11932
- Saitoh T, Fujita N, Hayashi T, et al. Atg9a controls dsDNA-driven dynamic translocation. Pnas. 2009:2-6.
- Correia S, Ventura S, Parkhouse RM. Identification and utility of innate immune system evasion mechanisms of ASFV. Virus Res. 2013; 173(1): 87-100. doi:10.1016/j.virusres.2012.10.013
- Li D, Yang W, Li L, et al. African Swine Fever Virus MGF-505-7R Negatively Regulates cGAS–STING-Mediated Signaling Pathway. J Immunol. 2021; 206(8): 1844-1857. doi:10.4049/jimmunol.2001110
- Yang K, Huang Q, Wang R, et al. African swine fever virus MGF505-11R inhibits type I interferon production by negatively regulating the cGAS-STING-mediated signaling pathway. Vet Microbiol. 2021; 263(July).
- Wang X, Wu J, Wu Y, et al. Inhibition of cGAS-STING-TBK1 signaling pathway by DP96R of ASFV China 2018/1. Biochem Biophys Res Commun. 2018; 506(3): 437-443. doi:10.1016/j.bbrc.2018.10.103
- Yang K, Xue Y, Niu H, et al. African swine fever virus MGF360 â 11L negatively regulates cGAS â STING â mediated inhibition of type I interferon production. Vet Res. 2022: 1-12. doi:10.1186/s13567-022-01025-0
- Wu S, Zhang Q, Zhang F, et al. HER2 recruits AKT1 to disrupt STING signalling and suppress antiviral defence and antitumour immunity. Nat Cell Biol. 2019; 21(8): 1027-1040. doi:10.1038/s41556-019-0352-z
- Liu X, Zhang K, Hu L, et al. African Swine Fever Virus pI215L Negatively Regulates cGAS-STING Signaling Pathway through Recruiting RNF138 to Inhibit K63-Linked Ubiquitination of TBK1. J Immunol. 2022; 207: 2754-2769. doi:10.4049/jimmunol.2100320
- Wang Y, Cui S, Xin T, et al. African Swine Fever Virus MGF360-14L Negatively Regulates Type I Interferon Signaling by Targeting IRF3. Front Cell Infect Microbiol. 2022; 11(January): 1-9. doi:10.3389/fcimb.2021.818969
- Liu H, Zhu Z, Feng T, et al. African Swine Fever Virus E120R Protein Inhibits Interferon Beta Production by Interacting with IRF3 To Block Its Activation. J Virol. 2021; 95(18): 1-17. doi:10.1128/jvi.00824-21
