Lupus Anticoagulant in Gulf War Illness and Autoimmune Disorders: A Common Pathway Toward Autoimmunity
Lisa M. James1,2,3, Rachel A. Johnson1, Scott M. Lewis1,4, Adam F. Carpenter1,4, Brian E. Engdahl1,2,5, Hollis E. Krug6,7, Apostolos P. Georgopoulos1,2,3,4*
1Brain Sciences Center, Department of Veterans Affairs Health Care System, Minneapolis, MN, 55417, USA
2Department of Neuroscience, University of Minnesota Medical School, Minneapolis, MN 55455, USA
3Department of Psychiatry, University of Minnesota Medical School, Minneapolis, MN 55455, USA
4Department of Neurology, University of Minnesota Medical School, Minneapolis, MN 55455, USA
5Department of Psychology, University of Minnesota Medical School, Minneapolis, MN 55455, USA
6Department of Rheumatology, Department of Veterans Affairs Health Care System, Minneapolis, MN, 55417, USA
7Department of Rheumatology, University of Minnesota Medical School, Minneapolis, MN 55455, USA
Abstract
Mounting evidence suggests that autoimmune mechanisms may underlie the chronic symptoms characteristic of Gulf War Illness (GWI). The presence of antiphospholipid antibodies including Lupus Anticoagulant (LA) are often associated with autoimmune disorders. Here we evaluated and compared blood samples from veterans with GWI and veterans with other autoimmune conditions including relapsing remitting multiple sclerosis, rheumatoid arthritis, Sjögren’s syndrome, and lupus for the presence of LA using Silica Clotting Time and dilute Russell’s Viper Venom Time assays. Positive LA was identified in one-quarter of veterans with GWI; this proportion was not statistically different from the proportion of positive LA identified in patients diagnosed with the other autoimmune conditions. The present findings add to the literature implicating autoimmune mechanisms in GWI and point to the presence of prothrombotic antiphospholipid antibodies as a common contributing factor in GWI and other autoimmune disorders. Furthermore, activation of the coagulation system suggests new potential avenues for treatment for LA-positive Gulf War veterans.
Introduction
Gulf War Illness (GWI) continues to plague one-third of veterans of the 1990-91 Persian Gulf War with chronic, debilitating symptoms affecting multiple organ systems. Symptoms range from fatigue, widespread pain, and cognitive problems to respiratory difficulties, dermatological complaints, and gastrointestinal issues1,2. Mounting evidence points to immune system disruptions3-7 and autoimmunity8-12 as mechanisms underlying the widespread symptoms characteristic of GWI. Evidence for autoimmunity in GWI includes brain functional patterns that are indistinguishable from those of known autoimmune disorders8, clinical and pathological features that are similar to other autoimmune syndromes9,11, and the presence of autoantibodies to neuronal and glial proteins12.
Although the cause of GWI is disputed, several research groups have suggested that immune system responses to foreign antigens such as pathogens, vaccines, and/or chemical/biological warfare agents (or prophylactics against them) may underlie GWI4,9-17. One theory has proposed that the immune response to such exposures may activate the coagulation system via the cross-reaction of antibodies against cell-surface antithrombotic proteins17. As theorized, evidence of blood hyper-coagulation was found in veterans with GWI17. That study also reported evidence of antiphospholipid antibodies (i.e., anti Beta-2-glycoprotein I; aβ2GPI) in some veterans with GWI. Antiphospholipid antibodies including aβ2GPI, anticardiolipin (aCL), and lupus anticoagulant (LA) are autoantibodies against phospholipids or phospholipid-binding proteins that result in formation of blood clots in vivo. Of the antiphospholipid antibodies, LA is the strongest risk factor for prothrombotic events18 and has been associated with increased risk of myocardial infarction19. Positive LA has been reported in patients with various autoimmune conditions including lupus (26-34%19,20), Sjögren’s syndrome (9-11%21,22), rheumatoid arthritis (6.6%23), and multiple sclerosis (22%24). Here we evaluate and compare the presence of positive lupus anticoagulant in GWI and four known autoimmune disorders to further characterize GWI with regard to autoimmune processes and inform potential treatment avenues.
Materials and Methods
A total of 148 veterans (126 men) participated in this study as paid volunteers. Participants belonged to one of the following 5 disease groups. (1) GWI without any autoimmune conditions (N = 84; 77 men); (2) relapsing remitting multiple sclerosis (RRMS; N = 18; 14 men); (3) rheumatoid arthritis (RA; N = 24; 22 men); (4) Sjögren’s syndrome (SS; N = 8; 4 men); and (5) lupus (Lupus; N = 14; 9 men). GWI patients met criteria for both Centers for Disease Control1 and Kansas2 case definitions. Diagnoses for autoimmune patient groups were established by specialists in the rheumatology or neurology clinics at the Minneapolis Veterans Affairs Health Care System. The mean ± SEM age (y) was 56.4 ± 0.90 for GWI, 45.3 ± 2.92 for RRMS, 66.5 ± 1.26 for RA, 58.0 ± 4.47 SS, and 62.3 ±2.29 Lupus. The Minneapolis VA Health Care System (VAHCS) Pathology and Laboratory Medicine Service evaluated blood samples for markers related to autoimmunity, including Lupus Anticoagulant (LA). LA was assessed using a standard Lupus Inhibitor Panel (VA Document ID:HEM02-023, issue date: 11/4/2019) consisting of Silica Clotting Time (SCT) and dilute Russell’s Viper Venom time (dRVVT), with both screen and confirm tests being run simultaneously for both tests. Cutoff values were 1.19 for SCT C test ratio and 1.15 for dRVVT C test ratio; the Lupus Inhibitor Panel outcome was Negative if both values were below cutoffs and Positive if either value was above the cutoff. Thus, for each group, the number of participants with positive LA test was available, from which the proportion of positives was calculated. This proportion of LA positive in the GWI group was compared to the corresponding proportions of the other groups using the Wald H0 test for independent-samples proportions (IBM-SPSS statistical package, version 27). Anticoagulants have been associated with false-positive lupus anticoagulant tests25; none of the participants in the present study were taking anticoagulants.
Each participant in the GWI group completed a standard GWI symptom questionnaire with six symptom domains2: fatigue, pain, neurological-cognitive-mood, gastrointestinal, respiratory, and skin. Individual symptom severity was reported in a scale from 0 to 3. For each participant, an average score per domain was calculated and used for comparisons between GWI participants that were LAC-positive or LAC-negative. using an independent samples t-test.
Results
Table 1 shows the proportions and associated statistics of LA observed in all disease groups (also see Figure 1). It can be seen that they were all similar, >25% for all but SS. Table 2 shows the results of testing these proportions between all disease pairs; none differed significantly from each other.
Table 1: Observed proportions and associated statistics of LA in the 5 disease groups.
|
|
N of LA present |
Total N |
Proportion |
Asymptotic Standard Error |
|
GWI |
22 |
84 |
0.262 |
0.048 |
|
RRMS |
6 |
18 |
0.333 |
0.111 |
|
RA |
8 |
24 |
0.333 |
0.096 |
|
SS |
1 |
8 |
0.125 |
0.117 |
|
Lupus |
4 |
14 |
0.286 |
0.121 |
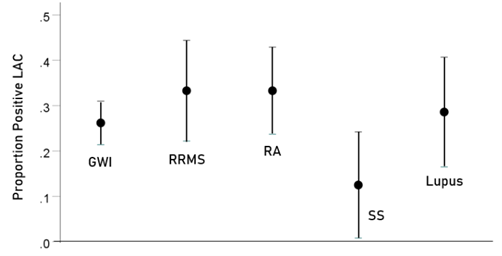
Figure 1: Proportion of positive LAC ± 1 standard error in each group
Table 2: Two-sided probability values of the Wald H0 test of the difference between two proportions.
|
|
GWI |
RRMS |
RA |
SS |
Lupus |
|
GWI |
X |
|
|
|
|
|
RRMS |
0.538 |
X |
|
|
|
|
RA |
0.491 |
1.000 |
X |
|
|
|
SS |
0.393 |
0.269 |
0.256 |
X |
|
|
Lupus |
0.852 |
0.773 |
0.761 |
0.387 |
X |
With respect to GWI symptom severity, no statistically significant differences were found for any of the 6 symptom domains above (P > 0.05 for all comparisons, uncorrected for multiple comparisons).
Discussion
In the present study we evaluated blood samples for the presence of positive LA in veterans with Gulf War Illness and compared the proportion of positive LA tests to that observed in other autoimmune disorders. The findings revealed the presence of positive LA in one-quarter of veterans with GWI, a rate that was comparable to that found in veterans diagnosed with autoimmune disorders. To our knowledge, this is the first study to demonstrate the presence of LA in Gulf War Illness, complementing findings of a previous study demonstrating the presence of another antiphospholipid antibody, B2GPI, in veterans with GWI17. The present findings add to the literature implicating autoimmune mechanisms in GWI and specifically point to the involvement of prothrombotic antiphospholipid autoantibodies in GWI.
Several researchers have characterized GWI as an autoimmune condition8-12. Environmental exposures to infectious agents26 and vaccines27 have been widely implicated in the development of various autoimmune conditions, and similarly, have been implicated in GWI9-11,14-16. Furthermore, several infectious agents28,29, including, most recently, SARS-CoV-230, and vaccines29 have been linked to the presence of antiphospholipid autoantibodies. In both infections and vaccines, phospholipid autoimmunity is purported to primarily occur as a result of molecular mimicry between phospholipid proteins and those of foreign antigens28,29. The extent to which Gulf War exposures (e.g., infectious agents, vaccines, chemical/biological warfare agents) may similarly share peptide sequences with phospholipids, thereby contributing to autoimmunity, remains to be investigated. In addition, chronic inflammation resulting from war-related environmental exposures, independently or coupled with stress, may result in tissue damage that, in turn, triggers autoimmunity. It is worth noting that the prevalence of lupus anticoagulant across all veteran patient groups in this study was somewhat higher here than what has previously been reported in the literature19-24. This raises the possibility that some environmental exposures that may contribute to autoimmunity are more common among the veteran population relative to their non-veteran counterparts.
The current finding that veterans with GWI and autoimmune disorders show comparable evidence of positive lupus anticoagulant further substantiates theories of GWI as an autoimmune condition8-11; however, only a subset of veterans with GWI (or other autoimmune disorders) in the current study exhibited positive LA, suggesting the influence of additional mechanisms, including potentially other immune-mediated processes. For example, GWI has been linked to lack of immunogenetic protection4 in which a mismatch between an individual’s human leukocyte antigen (HLA) composition and foreign antigens to which they are exposed (e.g., anthrax14,15) leads to persistent antigens that contribute to disease16,31. Of note, certain HLA alleles have also been associated with the presence of autoantibodies including lupus anticoagulant32. Future research evaluating the influence of HLA genotype on autoimmunity in GWI may prove informative with regard to understanding genetic susceptibility toward different manifestations of GWI.
Although the findings of the current study provide novel insights regarding autoimmune mechanisms associated with GWI, the findings must be considered within the context of study limitations. First, lupus anticoagulant was the only antiphospholipid antibody investigated in this study. With the exception of one prior study that reported positive B2GPI autoantibodies in three of the five GWI veterans that were evaluated17, the extent to which other antiphospholipid antibodies are present in these veterans, potentially contributing to autoimmunity, is unknown. Second, antiphospholipid antibody concentrations can fluctuate over time; however, these analyses were based on a single time point. Third, a veteran control group was not included in the present study, precluding comparison of LA-positivity in control veterans to the rates found in the present sample. Finally, this was a cross-sectional study. Longitudinal studies will be necessary to evaluate the long-term prognosis for Gulf War veterans with positive LA relative to those without positive LA. For instance, researchers have described a crescendo of autoimmunity characterized by increased concentrations of autoantibodies and increasingly varied antibodies in SLE33. It remains to be seen if a similar process is characteristic of LA-positive GWI.
Conclusion
The present findings suggest that for at least a non-trivial sub-population of veterans with GWI, activation of the coagulation system is an outcome that influences GWI and overlaps with known autoimmune disorders. In light of recent findings that male Gulf War veterans have significantly greater risk of stroke and myocardial infarction compared to the general population34, and increased risk of deep vein thrombosis/pulmonary embolism in female Gulf War veterans35, the current findings highlight the potential utility of routine monitoring for evidence of hypercoagulation in veterans with GWI and interventions aimed at reducing the likelihood of thrombotic events in LA-positive GWI veterans.
Acknowledgements:
This work was partially supported by the Department of Defense (Award Number W81XWH-15-1-0520), University of Minnesota (the American Legion Brain Sciences Chair) and the U.S. Department of Veterans Affairs. The sponsors had no role in the current study design, analysis or interpretation, or in the writing of this paper. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Author Contributions:
Contributed to data collection: AFC, LMJ, RAJ, SML. Contributed to participant recruitment and evaluation: BEE, LMJ, RAJ. Contributed to study design: APG, BEE, LMJ, AFC. Contributed to data analysis: APG. Wrote the paper: LMJ, APG. Contributed to editing the paper: All.
Conflicts of Interest:
None.
References
- Fukuda K, Nisenbaum R, Stewart G, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. 1998; 280(11): 981-988.
- Steele L. Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service Am J Epidemiol. 2000; 152: 992-1002.
- Broderick G, Kreitz A, Fuite J, et al. A pilot study of immune network remodeling under challenge in Gulf War Illness.Brain Behav Immunity. 2011; 25(2): 302–313.
- Georgopoulos AP, James LM, Mahan MY, et al. Reduced Human Leukocyte Antigen (HLA) protection in Gulf War Illness (GWI). EBioMedicine. 2016; 3: 79-85.
- Parkitny L, Middleton S, Baker K, et al. Evidence for abnormal cytokine expression in Gulf War Illness: A preliminary analysis of daily immune monitoring data. BMC Immunology. 2015; 16(1): 1-0.
- Skowera A, Hotopf M, Sawicka E, et al. Cellular immune activation in Gulf War veterans. J Clin Immunol. 2004; 24(1): 66-73.
- Whistler T, Fletcher MA, Lonergan W, et al. Impaired immune function in Gulf War illness. BMC Med Genomics. 2009; 2(1): 1-3.
- Georgopoulos AP, James LM, Carpenter AF, et al. Gulf War illness (GWI) as a neuroimmune disease. Exp Brain Res. 2017; 235(10): 3217-3225.
- Israeli E. Gulf War syndrome as a part of the autoimmune (autoinflammatory) syndrome induced by adjuvant (ASIA). Lupus. 2012; 21(2): 190-4.
- Moss JI. Gulf War illnesses are autoimmune illnesses caused by increased activity of the p38/MAPK pathway in CD4+ immune system cells, which was caused by nerve agent prophylaxis and adrenergic load. Med Hypotheses. 2013; 81(6): 1002-3.
- Shoenfeld Y, Agmon-Levin N. ‘ASIA’–autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011; 36(1): 4-8.
- Abou-Donia MB, Conboy LA, Kokkotou E, et al. Screening for novel central nervous system biomarkers in veterans with Gulf War Illness.Neurotoxicol Teratol.2017; 61: 36–46.
- Coughlin SS. A neuroimmune model of Gulf War Illness. J Environ Health Sci. 2017; 3: :10.15436/2378-6841.17.1665.
- Tsilibary E-PC, Souto EP, Kratzke M, et al. Anthrax Protective Antigen 63 (PA63): Toxic Effects in Neural Cultures and Role in Gulf War Illness (GWI).Neuroscience Insights. 2020; 15: 1-11.
- Tsilibary E-PC, Souto EP, Kratzke M, et al. Vaccine-induced adverse effects in cultured neuroblastoma 2A (N2A) cells duplicate toxicity of serum from patients with Gulf War Illness (GWI) and are prevented in the presence of specific anti-vaccine antibodies.Vaccines. 2020; 8(2): 232.
- James LM, Christova P, Engdahl BE, et al. Human leukocyte antigen (HLA) and Gulf War Illness (GWI): HLA-DRB1*13:02 spares subcortical atrophy in Gulf War veterans.EBioMedicine.2017;26: 126–131.
- Hannan KL, Berg DE, Baumzweiger W, et al. Activation of the coagulation system in Gulf War Illness: a potential pathophysiologic link with chronic fatigue syndrome A laboratory approach to diagnosis. Blood Coagul Fibrinolysis. 2000; 11(7): 673-8.
- Galli M, Luciani D, Bertolini G, et al. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature.Blood. 2003; 101: 1827–32.
- Petri M. Update on anti-phospholipid antibodies in SLE: the Hopkins’ Lupus Cohort.Lupus. 2010; 19(4): 419-423.
- Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders: prevalence and clinical significance. Ann Intern Med. 1990; 112(9): 682-98.
- Fauchais AL, Lambert M, Launay D, et al. Antiphospholipid antibodies in primary Sjögren's syndrome: prevalence and clinical significance in a series of 74 patients. Lupus. 2004; 13(4): 245-8.
- Pasoto SG, Chakkour HP, Natalino RR, et al. Lupus anticoagulant: a marker for stroke and venous thrombosis in primary Sjögren’s syndrome. Clinical Rheumatol. 2012; 31(9): 1331-8.
- Kim KJ, Baek IW, Park KS, et al. Association between antiphospholipid antibodies and arterial thrombosis in patients with rheumatoid arthritis. Lupus. 2017; 26(1): 88-94.
- Ijdo JW, Conti-Kelly AM, Greco P, et al. Anti-phospholipid antibodies in patients with multiple sclerosis and MS-like illnesses: MS or APS? Lupus. 1999; 8(2): 109-15.
- Tripodi A, Cohen H, Devreese KMJ. Lupus anticoagulant detection in anticoagulated patients. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020; 8: 1569–1575
- Smatti MK, Cyprian FS, Nasrallah GK, et al. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019; 11(8): 762.
- Agmon-Levin N, Paz Z, Israeli E, et al. Vaccines and autoimmunity. Nat Rev Rheumatol. 2009; 5(11): 648.
- Asherson RA, Cervera R. Antiphospholipid antibodies and infections. Ann Rheum Dis. 2003; 62(5): 388-93.
- Cruz-Tapias P, Blank M, Anaya JM, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012; 24(4): 389-93.
- Reyes Gil M, Barouqa M, Szymanski J, et al. Assessment of lupus anticoagulant positivity in patients with coronavirus disease 2019 (COVID-19).JAMA Netw Open.2020; 3(8): e2017539.
- James LM, Georgopoulos AP. Persistent antigens hypothesis: the human leukocyte antigen (HLA) connection. J Neurol Neuromed. 2018; 3(6): 27-31.
- Arnett FC, Olsen ML, Anderson KL, et al. Molecular analysis of major histocompatibility complex alleles associated with the lupus anticoagulant. J Clin Invest. 1991; 87(5): 1490-5.
- Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. NEJM. 2003; 349(16): 1526-33.
- Zundel CG, Krengel MH, Heeren T, et al. Rates of chronic medical conditions in 1991 Gulf War veterans compared to the general population. Int J Environ Res Pub He. 2019; 16(6): 949.
- Brown MC, Sims KJ, Gifford EJ, et al. Gender-based differences among 1990–1991 gulf war era veterans: Demographics, lifestyle behaviors, and health conditions. Women's Health Issues. 2019; 29: S47-55.
