Poor Prognosis in HBV-associated Hepatocellular Carcinoma After Successful Viral Suppression: A Case Series Highlighting a Need for a Cure
Salil Chowdhury1, Daniel Garrido2, Dina Halegoua-DeMarzio3, Christopher Roth4, Hie-Won Hann3*
1Thomas Jefferson University Hospital, Department of Internal Medicine, Philadelphia, PA
2Cooper University Hospital, Digestive Health Institute, Camden, NJ
3Thomas Jefferson University Hospital, Division of Gastroenterology and Hepatology, Philadelphia, PA
4Thomas Jefferson University Hospital, Department of Radiology, Philadelphia PA
Abstract
Hepatocellular Carcinoma (HCC) is one of the leading causes of cancer-related deaths worldwide, with chronic hepatitis B virus (HBV) infection being an important risk factor for HCC. While nucleos(t)ide analog (NA) therapy has succeeded in suppressing HBV replication and decreasing the risk of HCC in patients with HBV infection, there remains a persistent risk of HCC in those patients. Furthermore, previous studies have highlighted worse survival in patients who developed HCC while on successful NA therapy compared to those who developed HCC without previous NA treatment.
We conducted a long-term, retrospective case study in 5 patients observed between 10 and 25 years, to further explore the poor outcomes in patients with HBV-associated HCC with or without previous NA therapy. Our study highlights the aggression and recurrence of HCC in patients with HBV infection, well-suppressed on NA therapy. The results of our observation emphasize the need for early referral for liver transplantation in these select patients.
Introduction:
Since the discovery of hepatitis B virus (HBV) by Blumberg in 19651, it has presented a global health challenge. There are 296 million people living with chronic HBV infection as of 20192. HBV was also responsible for 820,000 deaths in 2019, mainly from cirrhosis and hepatocellular carcinoma (HCC), the latter of which being the leading cause2, 3. The incidence and mortality of HCC have been increasing in North America, as HCC has become the fastest growing cause of death related to cancer in the United States3. Following the development of the HBV vaccine, prenatal screening, and the effective anti-HBV drugs, the incidence of HBV-associated HCC has significantly decreased, currently accounting for 50% of HCC in the world4. However, there is still a significant population of chronically infected patients with HBV accounting for 600,000 deaths annually worldwide (with nearly 50% in China) and 10,0000 deaths annually in the United States4.
Nucleos(t)ide analog (NA) therapies, including lamivudine (LAM) in 1998, entecavir (ETV) in 2005, and tenofovir disoproxil fumarate (TDF) in 2008, have provided a breakthrough in the treatment of HBV infection, as they have demonstrated a reduction in the incidence of HCC5-8 and have become the standard of care9. Nonetheless, there remains a persistent risk of HCC in patients who are on NA therapy.
Multiple large and small studies have shown this persistent risk, even in patients achieving successful viral suppression on NA therapy9-15. Furthermore, a recent study showed that patients who develop HCC despite years of successful HBV suppression with NA therapy, carried worse prognoses than those who develop HCC with no previous NA treatment16.
Here, we present 5 cases which demonstrate particularly aggressive HCC that appeared after long term NA therapy. These cases include patients who were treatment experienced and those who were initially treatment naïve, received treatment for HCC and NA therapy, and later developed HCC despite successful viral suppression. Our observations further affirm the observed survival disparity and highlight the need for new treatment approaches including primary transplantation for HCC cases of treatment experienced patients.
Case 1:
A 65-year-old Asian American male, although perinatally infected with HBV, was found to be HBsAg positive at age 29. He presented to our institute at age 41 with chronic hepatitis B. He was started on LAM 150 mg daily. Excluding a short period of time off treatment after HBeAg seroconversion (per guidelines at the time), he remained on LAM and his HBV DNA has remained undetectable for the following 19 years. He has had routine abdominal imaging biannually and later annually. At age 65, his abdominal MRI showed increased echogenicity of the liver. Of note, the patient had been diagnosed with diabetes mellitus and receiving proper therapy. The following year, his abdominal MRI showed a 4.9 x 4.9 cm lesion in segment 4 (LI RADS 5), and tumor thrombosis in the right anterior portal vein, shown in Figure 1(c). His alanine aminotransferase (ALT) was 25 IU/L, platelets 192 B/l, hemoglobin A1C 6.2%, alpha-fetoprotein (AFP) 2.0 ng/ml, and creatinine 0.92 mg/dl. Chest CT scan showed no evidence of metastasis. He underwent Y90 trans-arterial radioembolization (TARE), and afterwards there was no longer tumor thrombus in the right portal vein. He is currently undergoing evaluation for liver transplantation.
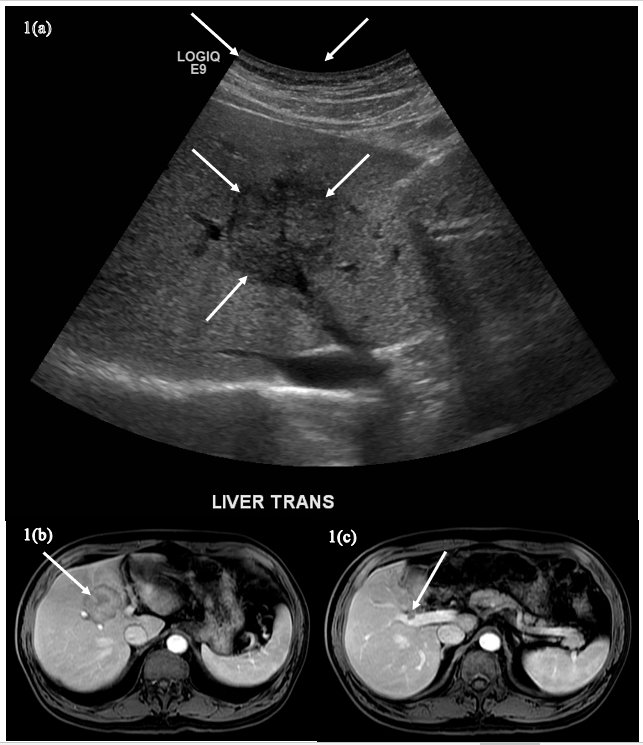
Figure 1(a)-(c): The transverse ultrasound image (A) shows a hypoechoic, solid-appearing lesion in the liver (arrows). The fat-suppressed, T1-weighted postcontrast image from an MRI obtained weeks after the ultrasound (B) shows a left lobar mass (arrow). The more caudal image (C) demonstrates portal venous tumor thrombus associated with the mass and typical of hepatocellular carcinoma with tumor in the vein (TIV) (arrow).
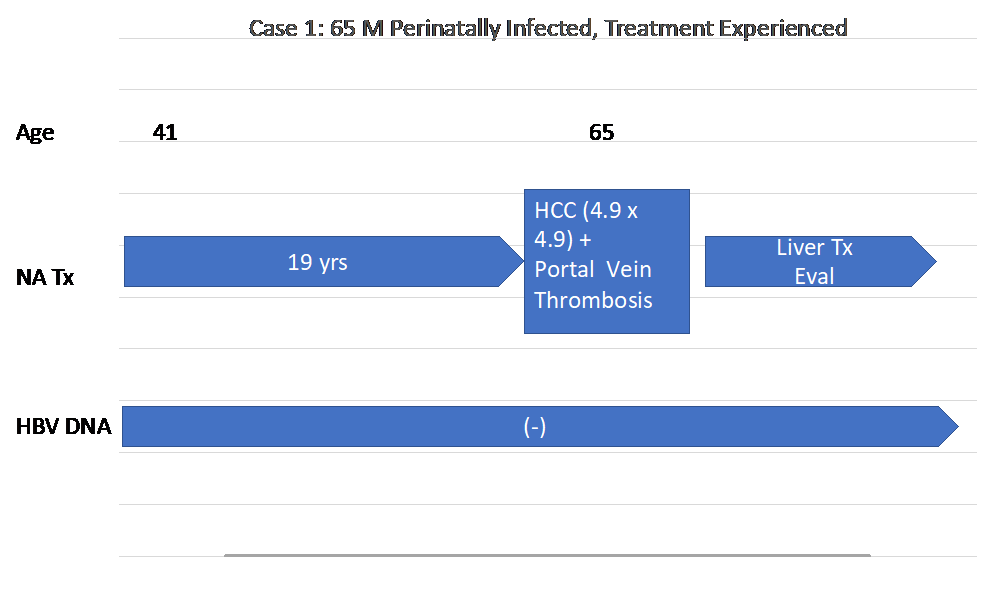
Figure 1(d): Timeline of Case 1’s history
Case 2
A 57-year-old Asian American male, although perinatally infected, was diagnosed with HBV infection after a presentation of bleeding esophageal varices. His mother had HBV-associated cirrhosis and died two weeks after receiving liver transplantation.
Upon his first visit to clinic, his HBV DNA was 9.4 x 106 copies/ml. He was started on LAM 150 mg daily followed later with the addition of TDF 300 mg. Within one year of treatment, his HBV DNA became undetectable, and remained undetectable for the next ten years. Annual and semiannual imaging showed stable cirrhosis on ultrasound and MRI. His AFP remained in the range of 4.0 ng/ml. He was continued on TDF as a single drug regimen for the next 5 years. Lately, AFP was elevated to 8.9 ng/ml followed by 9.5 ng/ml one month later. An abdominal MRI showed a 2.2 x 1.7 cm mass in Segment 4b (LI RADS 5) seen in Figures 2(a)-(c). Subsequent ultrasound-guided core biopsy confirmed HCC. He underwent laparoscopic microwave tumor ablation (MW). ETV 1 mg daily was added to his drug regimen given findings of HCC, and he was listed for liver transplantation. His AFP rose to 103 ng/ml while being evaluated for liver transplantation. A CT scan of his chest revealed multiple lung metastasis, shown in figures 2(d).
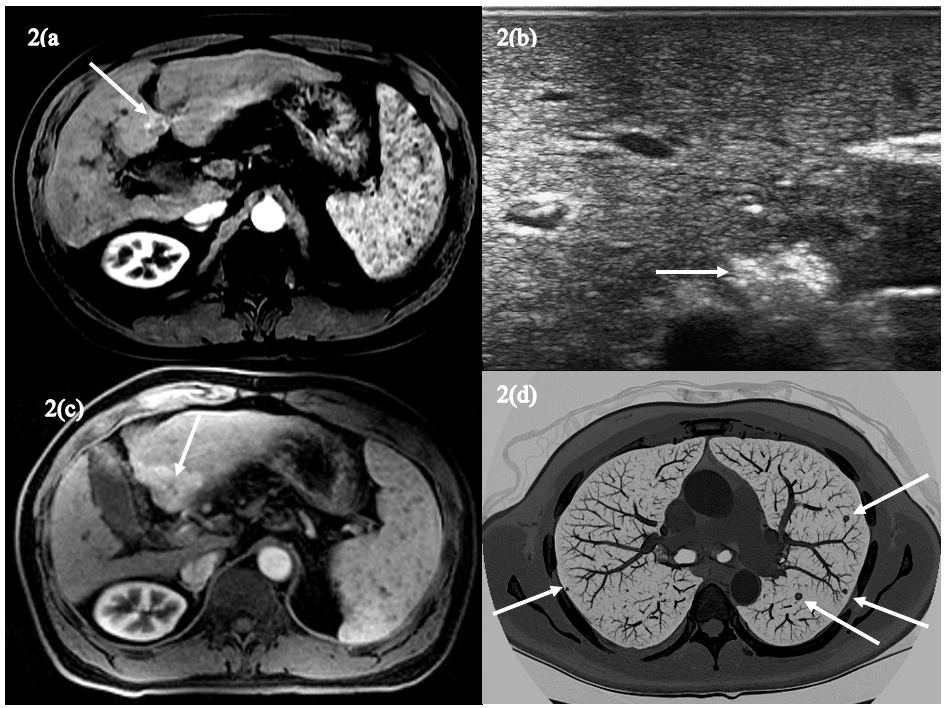
Figure 2(a)-(d): The axial fat-suppressed, T1-weighted arterial phase postcontrast image (A) shows an approximately 2cm hypointense lesion in segment 4B that demonstrates heterogeneous hyperenhancement (arrow). The ultrasound image following microwave ablation (B) shows increased echogenicity within the treated lesion (arrow) indicating treatment effects. The subsequent fat-suppressed, T1-weighted postcontrast arterial phase image (C) shows an approximately 4cm hyperenhancing LI-RADS 5 lesion (arrow) confirming the diagnosis of HCC. An axial maximal intensity projection (MIP) image (D) from a subsequent chest CT shows numerous pulmonary nodules (arrows) typical of metastases.
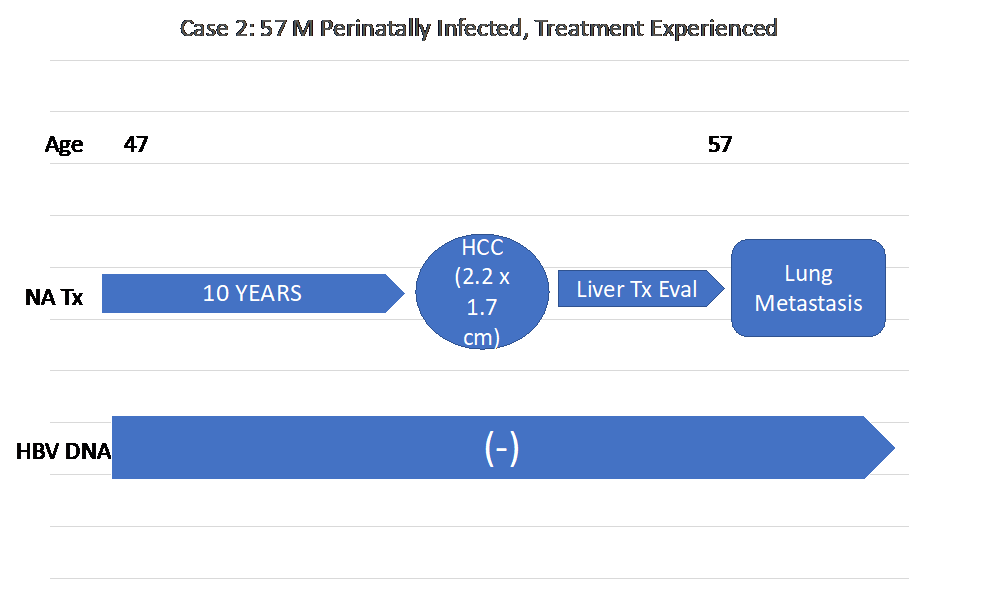
Figure 2(e): Timeline of Case 2’s history
Case 3:
A 64-year-old Asian American male, although perinatally infected, was noted to have chronic hepatitis B at age 37. His ALT was 198 IU/L, AST was 125 IU/L. Abdominal ultrasound was unremarkable. He was started on TDF 300 mg daily with normalization of his liver enzymes and undetectable HBV DNA. He was continued on TDF and his HBV DNA remained undetectable for the next ten years. An abdominal MRI done at this time, showed a 3 cm mass in the left lobe of his liver. He underwent local MW tumor ablation and was continued on TDF. A new lesion appeared 15 months later. He is currently listed for liver transplantation.
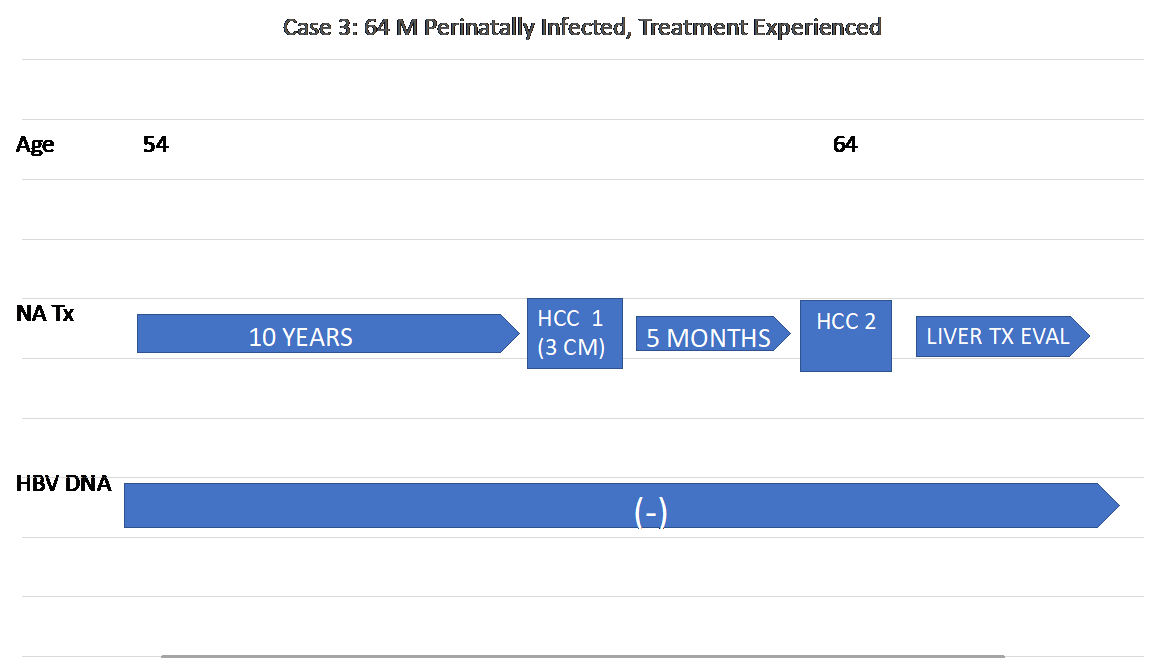
Figure 3(a): Timeline of Case 3’s history
Case 4:
An 84-year-old Asian American female was found to be HBsAg positive at age 53. She moved away and returned 10 years later (in 2000) at age 64 for a follow up visit. Abdominal MRI showed a 2.1 x 5 cm mass suggestive of HCC. Her AFP was 6.7 ng/ml. Other notable labs also included an albumin 4 g/dL, and platelets of 122,000 B/L. She underwent radiofrequency tumor ablation (RFA) of her tumor, followed by trans-arterial chemoembolization (TACE) for residual tumor. She was started on LAM 150 mg daily, later switched to TDF 300 mg, and became HBV DNA negative, which she remained throughout follow up. Five years later, she was found to have recurrence at her treated site and a second, new lesion on abdominal MRI consistent with HCC and underwent TACE. Ten years after this recurrence, MRI revealed another 5 mm lesion in segment 2, initially read as LI-RADS 4. This was monitored every four to six months, with slow growth, as well as evidence of regression. However, on one abdominal MRI two years afterwards, the tumor had grown to 1.2 cm, 28 years after the development of her first HCC. At age 84, she is now arranged to receive ablation of her tumor in her home state.
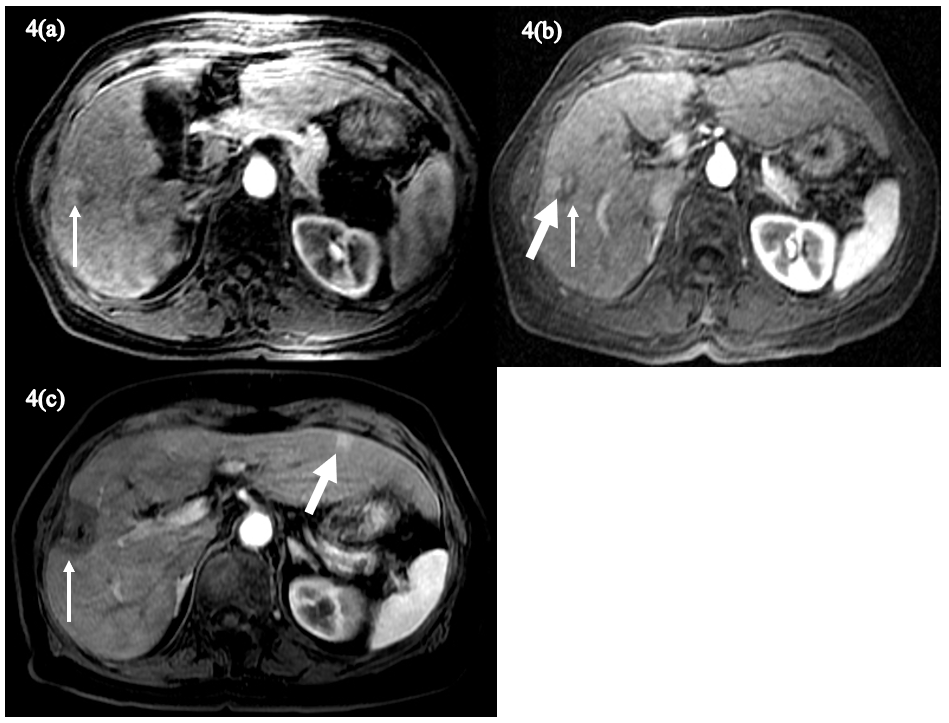
Figure 4(a)-(c): The axial fat-suppressed, T1-weighted postcontrast image (A) shows a 2cm heterogeneously hyperenhancing lesion in segment 5 (arrow). Over 3 years following transarterial chemoembolization (TACE), on the fat-suppressed, T1-weighted postcontrast image (B) the treated lesion (arrow) exhibits heterogeneous signal corresponding to posttreatment changes, although there is focal hyperenhancement adjacent to the treated lesion (thick arrow). The axial fat-suppressed, T1-weighted postcontrast image (C) obtained 14 years following repeat TACE and microwave ablation shows absent enhancement in the treated area in segment 5 (arrow) with a new hyperenhancing lesion in segment 3 (thick arrow).
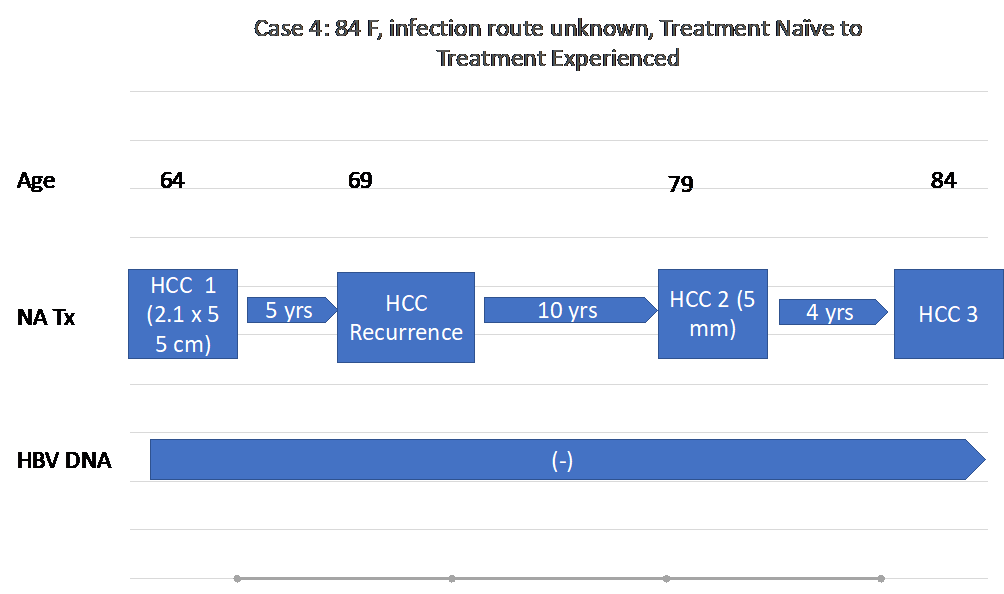
Figure 4D: A timeline of Case 4’s history
Case 5:
An 83-year-old Asian American male was noted to be HBsAg positive at age 57. He was followed annually, without treatment, and notably had normal ALT and low platelets around 100,000 B/L at age 65. At age 68, during annual imaging, an MRI of his abdomen showed a cirrhotic liver with a 1.0 cm mass in the right lobe of his liver consistent with HCC. His HBV DNA was 10.3 x 107 copies/ml, his ALT was 98 IU/L, his platelets 96,000 B/L, and AFP 12.5 ng/ml. He was started on LAM 150 mg and TDF 300 mg daily. He underwent RFA, continued on TDF, and was followed with lab work and imaging annually in his hometown. At age 79, 11 years after his initial diagnosis of HCC, two new lesions were found on abdominal MRI and ultrasound measuring 3 x 2.8 cm and 2 x 1.7 cm in segment 5 adjacent to the gallbladder, and segment 7/8 respectively. His AFP at the time was 2.9 ng/ml, while his HBV DNA was undetectable. He underwent laparoscopic cholecystectomy and tumor ablation in both segments. Four years after this ablation, at age 83, he was found to have a new 4.8 cm lesion in segment 4 on abdominal MRI and underwent Y90 embolization, shown in Figure 5(d).
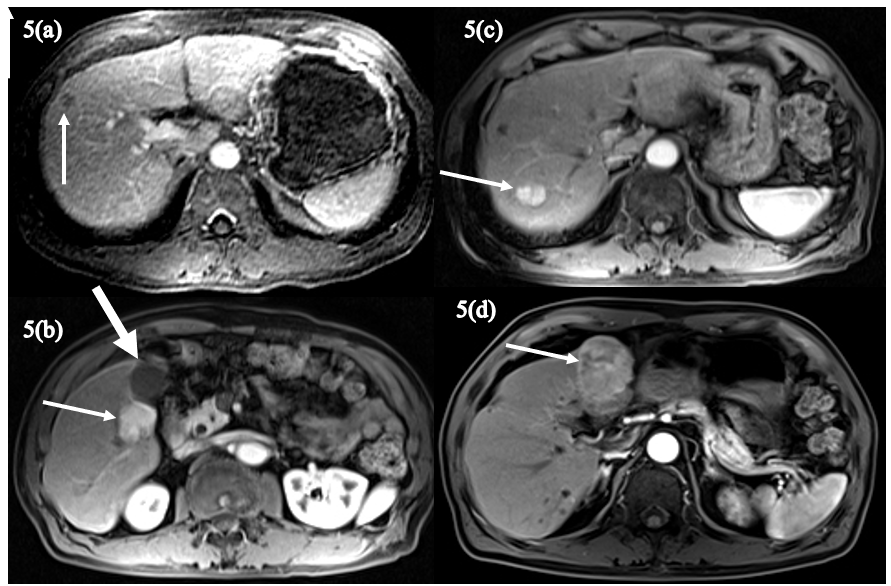
Figure 5(a)-(d): The axial fat-suppressed, T1-weighted delayed postcontrast image (A) shows a small hepatic segment 8 lesion exhibiting washout (arrow) and corresponding to a small HCC. The axial fat-suppressed, T1-weighted arterial phase postcontrast image obtained 11 years later (B) shows a 3cm hyperenhancing lesion (arrow) in segment 5 adjacent to the gallbladder (thick arrow) indicating recurrent HCC. On a more cephalad arterial phase image (C), a second hyperenhancing HCC (arrow) in segment 7 is evident. Four years later, the fat-suppressed, T1-weighted arterial phase postcontrast image (D) shows yet another large, hyperenhancing segment 4 lesion (arrow) consistent with HCC.
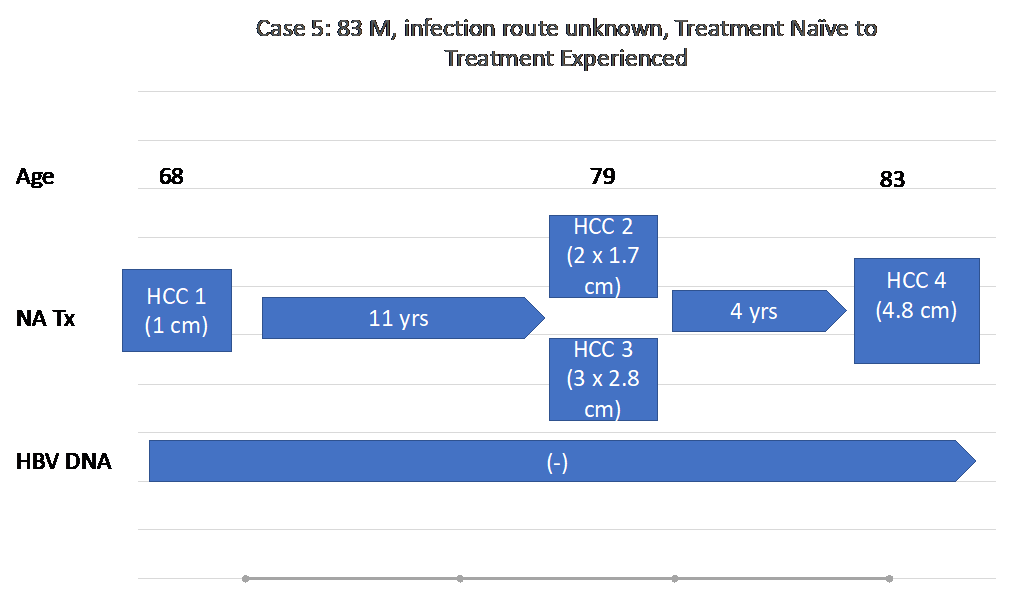
Figure 5(e): A timeline of Case 5’s history.
Discussion
The persistent risk for the development of HCC despite successful HBV suppression has been reported in the past9-17. There has been limited exploration into the nature of HCC in those who are being treated with NA therapy. In their earlier case study, Garrido et al observed that among patients with HBV infection, those who developed HCC with no previous NA therapy had better survival outcomes than those who developed HCC despite successful NA therapy16. These findings posed a question regarding the aggressive nature of certain HCC cases that breakthrough despite successful viral suppression. In our case series, we present five longitudinal cases, three (cases 1-3) who had been treated with NA therapy and developed HCC, and two (cases 4, 5) who had been treatment naïve prior to development of their first HCC and treatment experienced after development of their second or recurrent HCC. Our observation period for the treatment naïve HCC patients reaches 24 years for case 1, and 10 years for cases 2 and 3. The observation period for the HCC patients who were treatment naïve and later treatment experienced reaches 20 years and 15 years for cases 4 and 5 respectively.
Our studies clearly illustrate, not only the persistent risk of HCC in those with seronegative HBV DNA under treatment with NA therapy, but also the aggressive and evolving nature of these tumors. In two of these cases (cases 4 and 5), in which our patients developed tumors both as treatment naïve patients and treatment-experienced patients, we observed the accelerated progression and recurrence of tumors after becoming treatment experienced.
While the potential etiology for the aggressive nature of these new HCCs remains unclear, one must consider the HBV replication cycle16, 18. HBV is an enveloped, partially double-stranded virus. As it enters the host cell, its DNA converts from its relaxed circular shape into a covalently closed circular DNA (cccDNA)15. cccDNA remains in the infected hepatocyte nuclei and acts a template for viral replication. It is believed that cccDNA is the reservoir for chronic HBV infection and recurrence19 and the predominant form of HBV DNA in tumor tissue20. Integration of HBV DNA into infected hepatocytes, which follows, is important to carcinogenesis due to promotion of DNA instability and generation of new mutations18, 21. All the current NA analogs only act on DNA polymerase and prevent reverse transcription only providing a functional cure for the disease22.
Regarding the poor prognosis seen in patients developing HCC while on NA therapy, one potential mechanism may lie in cccDNA. Zhou et al postulated that drug-resistance mutations may also be harbored in cccDNA, as this was seen in their woodchuck model23. Exogenous factors including current NA agents present selection pressures on the virus, which may drive mutagenesis. As cccDNA is maintained in both the hepatocyte and tumor cell, it could be plausible that this provides a reservoir for instability and mutagenesis in these cells due to these selection pressures, especially in those currently on NA therapy18, 24. The effects of this are unknown but may be connected to this aggressive presentation of HCC.
Viral integration into the host genome is an important part of the oncogenic process in HBV-mediated HCC as well25, as described above. Wooddell et al demonstrated that integrated DNA was an under-accounted for source of HBV transcription in humans treated with NAs and represented the dominant source of transcripts in chimpanzees, unchanged by NA treatment26. Persistence of this integrated DNA in NA treated patients, has the potential to further drive mutagenesis and chromosomal instability, which may explain the recurrence of aggression of this disease. Further genomic analysis and precision medicine may give us insight into biomarkers to screen patients at increased risk for HCC18, 25.
However, only a true cure, targeting the above mechanisms can eliminate the persistent risk of HCC in patients who have been infected with HBV. Various targets for cccDNA focused therapy include genetic editing technology aimed at degrading such as CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) or other effective nucleases which have shown effect in vitro27. Interferons and cytokines have also shown promise in inhibiting transcription of cccDNA27-29. Otherwise, investigation is underway into the efficacy of various newer agents which target integrated DNA as well as cccDNA, including small molecule DNA and RNA silencers27.
We realized from the small observation that progression to HCC despite successful viral suppression still carries significant risk for decompensation. This should emphasize the role for early referral and discussion with patients about the need for liver transplantation given increased mortality.
Conclusion:
The case series presented here gives a unique and longitudinal insight into patients who develop HCC while receiving NA therapy. Further explored in our series, is the aggression and recurrence of HCC in patients who are treatment experienced. Without a true cure for HBV, one that addresses the persistence of cccDNA and integrated viral DNA, the risk of HCC remains even in patients who have well-suppressed HBV replication on NA therapy. Further research and investigation are necessary in the exploration of our findings.
Conflict of Interest:
HW Hann: Serves the National Advisory Board of The Gilead Sciences. Receives grant from Gilead and Assembly Biosciences. All other authors declared that there are no conflicts of interest.
References
- Blumberg BS, Alter HJ, Visnich S. A "New" Antigen in Leukemia Sera. JAMA. 1965; 191: 541-6.
- 2021; Pages https://www.who.int/news-room/fact-sheets/detail/hepatitis-b on 5/17/2022 2022.
- Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019; 156(2): 477-91 e1.
- Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006; 45(4): 529-38.
- Hosaka T, Suzuki F, Kobayashi M, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013; 58(1): 98-107.
- Kim WR, Loomba R, Berg T, et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer. 2015; 121(20): 3631-8.
- Sung JJ, Tsoi KK, Wong VW, et al. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2008; 28(9): 1067-77.
- Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004; 351(15): 1521-31.
- Vlachogiannakos J, Papatheodoridis G. Hepatocellular carcinoma in chronic hepatitis B patients under antiviral therapy. World J Gastroenterol. 2013; 19(47): 8822-30.
- Papatheodoridis GV, Lampertico P, Manolakopoulos S, et al. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010; 53(2): 348-56.
- Papatheodoridis GV, Manolakopoulos S, Touloumi G, et al. Virological suppression does not prevent the development of hepatocellular carcinoma in HBeAg-negative chronic hepatitis B patients with cirrhosis receiving oral antiviral(s) starting with lamivudine monotherapy: results of the nationwide HEPNET. Greece cohort study. Gut. 2011; 60(8): 1109-16.
- Dargan A, Wong SY, Coben R, et al. Persistent risk for hepatocellular carcinoma after more than a decade of successful hepatitis B virus suppression. Minerva Gastroenterol Dietol. 2017; 63(1): 74-6.
- Yoo J, Hann HW, Coben R, et al. Update Treatment for HBV Infection and Persistent Risk for Hepatocellular Carcinoma: Prospect for an HBV Cure. Diseases. 2018; 6(2).
- Shinn BJ, Martin A, Coben RM, et al. Persistent risk for new, subsequent new and recurrent hepatocellular carcinoma despite successful anti-hepatitis B virus therapy and tumor ablation: The need for hepatitis B virus cure. World J Hepatol. 2019; 11(1): 65-73.
- Boortalary T SB, Coben R, Conn M, et al. Are We Close to Achieving a HBV Cure? Risk for Hepatocellular Carcinoma Persists Despite Long-term HBV Suppression: An Update on Our Experience. Archives of Gastroenterology. 2020; 1(4).
- Garrido D, Block, Peter, et al. Survival Disparity Between Antiviral-Treated and Antiviral-Naâve Patients Who Develop Their First HBV-Associated Hepatocellular Carcinoma. Archives of Gastroenterology. 2021; 2(3).
- Shinn BJ, Kistler C, Roth C, et al. Need For HBV Cure: Persistent Risk For Subsequent New And Recurrent HCC Even After A Decade Of Successful Anti-HBV Therapy And Initial Tumor Ablation. Archives in Cancer Research. 2018; 06(02).
- Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016; 64(1 Suppl): S84-S101.
- Faria LC, Gigou M, Roque-Afonso AM, et al. Hepatocellular carcinoma is associated with an increased risk of hepatitis B virus recurrence after liver transplantation. Gastroenterology. 2008; 134(7): 1890-9; quiz 2155.
- Wong DK, Yuen MF, Poon RT, et al. Quantification of hepatitis B virus covalently closed circular DNA in patients with hepatocellular carcinoma. J Hepatol. 2006; 45(4): 553-9.
- Tan YJ. Hepatitis B virus infection and the risk of hepatocellular carcinoma. World J Gastroenterol. 2011; 17(44): 4853-7.
- Levrero M, Testoni B, Zoulim F. HBV cure: why, how, when? Curr Opin Virol. 2016; 18: 135-43.
- Zhou T, Saputelli J, Aldrich CE, et al. Emergence of drug-resistant populations of woodchuck hepatitis virus in woodchucks treated with the antiviral nucleoside lamivudine. Antimicrob Agents Chemother. 1999; 43(8): 1947-54.
- Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology. 2009;137(5): 1593-608 e1-2.
- Lin SY, Zhang A, Lian J, et al. Recurrent HBV Integration Targets as Potential Drivers in Hepatocellular Carcinoma. Cells. 2021; 10(6).
- Wooddell CI, Yuen MF, Chan HL, et al. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med. 2017; 9(409).
- Tsounis EP, Tourkochristou E, Mouzaki A, et al. Toward a new era of hepatitis B virus therapeutics: The pursuit of a functional cure. World J Gastroenterol. 2021; 27(21): 2727-57.
- Lucifora J, Protzer U. Attacking hepatitis B virus cccDNA--The holy grail to hepatitis B cure. J Hepatol. 2016; 64(1 Suppl): S41-S8.
- Palumbo GA, Scisciani C, Pediconi N, et al. IL6 Inhibits HBV Transcription by Targeting the Epigenetic Control of the Nuclear cccDNA Minichromosome. PLoS One. 2015; 10(11): e0142599.
