Serologic Response of SARS-CoV-2 mRNA-based Vaccines in Patients with Autoimmune Diseases
Kushal Gandhi1#, Nathan Joshua Manales1#, Asley Sanchez1#, Srikanth Mukkera1*, Anusha Ammu1, Janine Klar1, Alex Gibson1, Evangelina Santiago2, Ailena Mulkey2, Jammie Holland2, Maneesh Mannem1, Lakshmi P. Alahari1, and John Garza1,3
1 School of Medicine, Texas Tech University Health Sciences Center (TTUHSC) at the Permian Basin, TX, USA
2 Clinical Research institute at the Permian Basin, Texas Tech University Health Sciences Center – Permian Basin, TX, USA
3 The University of Texas Permian Basin, Odessa, TX, USA
# Authors contributed equally
Abstract
Background: In the spring of 2021, coronavirus disease 2019 (COVID-19) vaccines were approved and distributed in the United States for the public to combat the COVID-19 pandemic, but their rapid development leaves some questions unanswered. Vaccine efficacy has always been a point of interest for individuals with rheumatological diseases that take immunosuppressants. This study investigates the vaccine efficacy of two COVID-19 mRNA-based vaccines, Moderna and Pfizer, in subjects in West Texas patients with autoimmune diseases.
Materials and Methods: Blood was collected from Texas Tech University employees who received both doses of COVID-19 vaccines within the past nine months. Subjects were separated into either a group with a known history of rheumatic disease (n=18) or those without (n=18). The samples were analyzed for serum immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM) levels using specific enzyme-linked immunoassay kits, and a neutralizing antibody test using a surrogate virus was conducted as well. Results were analyzed using the Mann-Whitney U test (unpaired, two-tailed).
Results: There was no significant difference in serum IgG and IgA levels between the control and rheumatologic disease groups, but there were significant differences in serum IgM levels. All subjects cleared the threshold for the neutralizing antibody test.
Conclusion: The relatively similar serum IgG levels and the 100% detection rate of effective neutralizing antibodies across both groups indicate promising signs of serological response for subjects with autoimmune conditions, but the relatively low serum IgA and IgM levels of the study the group warrants further investigation.
Introduction
Two years after it was declared a global pandemic on March 11, 2020, coronavirus disease 2019 (COVID-19) remains a global struggle as it has proved to be challenging for even the most durable healthcare systems to handle, especially during its peaks. As of February 22, 2022, there have been 422 million cases of COVID-19 and the disease is responsible for 6 million deaths worldwide1. Like any other viral infection, COVID-19 has shown to trigger a number of autoimmune cell-mediated responses and auto-inflammatory sequelae. Infection in people with underlying autoimmune disorders has a lot of unpredictable interactions which are currently beyond rheumatologists’ expectations. Patients infected with COVID-19 may present with symptoms that are similar to influenza such as coughing, sore throat, fever, shortness of breath, muscle aches, fatigue, runny nose, congestion, nausea, abdominal pain, and loss of smell/taste as a unique feature of the virus. With later, more severe symptoms of being severe pneumonia, multiple organ failure, and acute respiratory distress syndrome (ARDS)2, 3. The main transmission route for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is human-to-human transmission via respiratory droplets, with incubation time of 2-14 days4. The health risk factors for COVID-19 are age and comorbidities, including hypertension, diabetes, coronary heart disease, etc.4, 5.
There is a lack of effectiveness and definite treatments for COVID-19 at present. Individuals afflicted with severe instances of the disease have continued to increase worldwide. Several drugs such as remdesivir, hydroxychloroquine, azithromycin, glucocorticoids, tocilizumab, lopinavir/ritonavir, favipiravir, monoclonal antibodies, and protease inhibitors have been used in clinical trials and but none of them have proven to be a definite treatment for COVID-196-8. A large global effort accelerated the development of effective vaccines; since then, many nations have prioritized the widespread deployment of COVID-19 vaccines. Currently, the types of COVID-19 vaccines produced by various pharmaceutical and biotechnological companies include live-attenuated vaccine, inactivated virus vaccine, subunit vaccine, viral vector-based vaccine, DNA vaccine, and RNA vaccine7.
Food and Drug Administration (FDA) approved two SARS-CoV-2 mRNA-based vaccines under emergency use authorization in December 2020.
Vaccine 1: Pfizer
Vaccine 2: Moderna
Both vaccines offer around 94%-95% of protection against COVID-19 if everyone vaccinates with two doses in 21-28 days. In the FDA’s approval of these vaccines for emergency use, they did not specify how these vaccines would affect individuals with autoimmune diseases who are taking immunosuppressive agents9. The American College of Rheumatology (ACR), a leader in education on autoimmune diseases, has recommended SARS-CoV-2 mRNA-based vaccines (Pfizer or Moderna) for patients with autoimmune diseases despite the fact that there is no data currently available on the risks of taking the vaccine since they are considered to be more at risk of severe COVID-19 complications than the general population in light of the pandemic9-11. Additionally, the Center for Disease Control and Prevention (CDC) recommends a booster dose of the COVID-19 vaccine in moderately or severely immunocompromised people in ages 12 years and older who received an mRNA COVID-19 vaccine primary series and an additional primary mRNA vaccine dose; of least 5 months after completing their primary dose.
Autoimmune disease flare-ups can happen after vaccination due to possible cross-reactivity and enigmatic inflammatory conditions12, 13. Poor serological response to vaccination is possible due to ongoing immunosuppressive therapy14. The documentation of antibody response toward SARS-CoV-2 infection is a crucial piece of the puzzle, and the amount of serological data available is rapidly increasing. SARS-CoV-2 infection follows a typical viral response pattern that starts with IgM first, followed closely by Immunoglobulin A (IgA), which peaks at two-three weeks post-symptom onset (PSO) before declining, and finally with Immunoglobulin G (IgG) antibodies that remain detectable for several months PSO. The novel mRNA vaccines developed by Pfizer-BioNTech and Moderna make up the overwhelming majority of the COVID-19 vaccines in the USA. Both vaccines have been reported to elicit a TH1 T cell response with minimal TH2 cytokine expression, and while there are several detailed studies analyzing IgG, the literature does not provide sufficient data regarding Immunoglobulin M (IgM)/IgA and how they behave in response to these mRNA-based vaccines15-17.
The purpose of our study is to see how the population of autoimmune disease patients was affected by the two doses of mRNA vaccines currently available in the USA. Our study will provide knowledge on how individuals with autoimmune diseases currently respond to the vaccines and guide future research to further study vaccine-related benefits in this subpopulation of individuals. This study aims to determine the serological response (particularly IgG, IgM, and IgA antibodies) of the SARS-CoV-2 mRNA-based vaccine in patients with a rheumatic disease.
Materials and Methods
Recruitment of participants and blood collection
The Institutional Review Board (IRB) approved our study (IRB protocol # A21-4231) which involved the employees from the Texas Tech University Health Sciences Center (TTUHSC) at the Permian Basin campus and Lubbock campus as well as Medical Center Hospital (MCH) at Odessa, Texas. The employees were surveyed in order to gauge interest in our research study using a Qualtrics survey. We asked potential participants to provide their contact information for further inquiry.
The participants interested were then contacted by our research team to answer a questionnaire and collection of blood to determine their serologic response after administration of SARS-CoV-2 mRNA-based vaccines (Pfizer or Moderna). The inclusion criteria for the study group were that the participants should have a known history of a rheumatologic disease, must have received both doses of the SARS-CoV-2 mRNA-based vaccines within the last 9 months, and must provide their proof of vaccination. Total of 18 participants in the control group (participants without rheumatologic/autoimmune disease) and 20 participants in the study group (participants with rheumatologic/autoimmune disease) were enrolled in our research study. Two participants from the control group decided to withdraw from our research study.
Enzyme-linked Immunoassay (ELISA) assay for IgG, IgM, and IgA antibodies
All serum samples and controls were taken out from cold storage (-80°C) and kept at room temperature before use. 100 µl of samples and positive control were added into the corresponding wells of the coated plate. The plate was covered with the plate sealer and incubated at room temperature for 60 minutes with gentle shaking. After incubation, the plate sealer was removed, and the plate was washed four times with 300 µl of wash solution (1X). 100 µl of prepared Biotinylated Anti-Human IgG (Cat# IEQ-CoVS1RBD-IgG, RayBiotech Life Inc., USA), IgM (Cat# IEQ-CoVS1RBD-IgM, RayBiotech Life Inc., USA), and IgA (Cat# IEQ-CoVS1RBD-IgA, RayBiotech Life Inc., USA) antibody solution was added to each well of the plate and incubated at room temperature for 30 minutes with gentle shaking. After incubation, the plate sealer was removed, and the plate was washed four times with 300 µl of wash solution (1X). 100 µl of TMB (3,3’,5,5’ tetramethylbenzidine dihydrochloride) one-step substrate reagent was added to each well of the plate and incubated in the dark at room temperature for 15 minutes with gentle shaking. 50 µl of stop solution was added to each well of the plate and the absorbance at 450 nm was read in a microplate reader.
Surrogate virus neutralization test
The SARS-CoV-2 sVNT Kit (Cat. No. L00847-A, Lot No. A210501, Genscript Biotech Corporation, Piscataway, NJ, USA), is a blocking ELISA detection tool. The kit was designed to mimic the neutralization process by incorporating two key components: the SARS-CoV-2 RBD Fragment conjugated to recombinant Horseradish peroxidase (HRP-RBD) and the human ACE2 receptor protein (hACE2). This protein-protein interaction between HRP-RBD and hACE2 can be blocked by neutralizing antibodies against the SARS-CoV-2 RBD in vaccinated individuals.
The positive and negative cutoffs for SARS-CoV-2 neutralizing antibodies can be interpreted by calculating the inhibition rate. The inhibition rate was calculated as follows (provided by Genescript) using the measured optical densities (OD) of each sample after evaluation of their sera:
The cutoff value is based on validation of the company’s panel of confirmed COVID-19 patient sera and healthy control sera. All serum or plasma samples and controls were taken out from cold storage (- 80â) and kept at room temperature before use. An amount of 100 µl of samples, positive control, and negative control was added into the corresponding wells of the coated plate. The capture plate was covered with the plate sealer and incubated at 37 °C for 60 minutes. The plate sealer was removed, and the plate was washed three times with 300 µl of wash solution (1X). 100 µl of TMB solution was added to each well of the plate and incubated in the dark at 37 °C for 10 minutes. 50 µl of stop solution was added to each well of the plate and the absorbance at 450 nm was read in a microplate reader.
Statistical analysis
The sample size for our research study was determined based on the general population in West Texas, USA, suffering from autoimmune disease. We analyzed the correlation between antibody responses (IgG, IgM, and IgA) detected by ELISA assay between the control and the study groups using Mann-Whitney U test (unpaired, two-tailed). Differences in neutralization assay between the control and the study groups were determined using Mann-Whitney U test (unpaired, two-tailed). Statistical analyses were conducted in Prism (GraphPad, version 9.3.1), a significance level of α was 0.05.
Results
Demographic characteristics
We prospectively enrolled 36 subjects for this research study in which 18 subjects were RA patients and 18 subjects were controlled. Demographic characteristics of the participants from both control and study groups were summarized in table 1. The average age of the subject in the control group was 30 years whereas in the study group was 34.5 years. In the control group, the Hispanic population was significantly higher compared to other race populations, whereas the white population was significantly higher in the study group (Table 1). In the control group, the majority of subjects had Pfizer vaccine; however, the majority of subjects in the study group had Moderna vaccine (Table 1). 50% and 80% of the subjects in the control and study groups reported minor vaccine side effects (fever, headache, fatigue, body aches, arm swelling and pain at the injection site, chills, nausea) respectively. Only 25% of the subjects in the study group reported flare-ups (swelling and painful throbbing at the joints, increased fatigue and general malaise, calor at the joints, increase in psoriasis, and asthma symptoms following administration of the vaccine). None of the subjects in the control (except one subject) and the study groups were diagnosed with COVID-19 infection after vaccination.
Table 1: The reported demographic results from the participant survey in both the control and study groups
|
|
Control (n=18) |
Study (n=18) |
|
|
Ethnicity |
|
|
American Indian or Alaskan Native
|
1 (5.6%) |
0 |
|
African American |
2 (11.1%) |
0 |
|
White |
5 (27.8%) |
10 (55.6%) |
|
Hispanic |
10 (55.5%) |
8 (44.4%) |
|
|
SARS-CoV-2 Vaccine Administered |
|
|
Moderna |
7 (39%) |
15 (83.3%) |
|
Pfizer |
11 (61%) |
3 (16.7%) |
|
|
Reported Minor Side Effects* |
|
|
Yes |
12 (67%) |
14 (77.7%) |
|
No |
6 (33%) |
4 (33.3%) |
|
|
Reported Flare Ups** |
|
|
Yes |
N/A |
5 (27.8%) |
|
No |
N/A |
13 (72.8%) |
|
|
Distribution of Autoimmune Diseases |
|
|
Ankolysing Spondylosis |
N/A |
2 (11.1%) |
|
Hashimoto’s Thyroiditis |
N/A |
1 (5.6%) |
|
Multiple Sclerosis |
N/A |
1 (5.6%) |
|
Psoriasis/Psoriatic Arthritis |
N/A |
5 (27.8%) |
|
Rheumatoid Arthritis |
N/A |
6 (33.3%) |
|
Sjörgren’s Syndrome |
N/A |
1 (5.6%) |
|
Systemic Lupus Erythematous
|
N/A |
1 (5.6%) |
|
Ulcerative Colitis |
N/A |
1 (5.6%) |
|
|
Medication Usage at the Time of Vaccination |
|
|
Yes |
N/A |
12 (66.7%) |
|
No |
N/A |
6 (33.3%) |
*Side effects reported include fever, headache, fatigue, body aches, arm swelling and/or pain at injection site, chills, nausea
**Flare-up symptoms reported include swelling and painful throbbing at the joints, increased fatigue and general malaise, calor at the joints, and an increase in psoriasis and asthma symptoms following administration of the vaccine.
aHispanic population was significantly higher in the control group, whereas the white population was significantly higher in the study group. Majority of subjects in the control group had Pfizer vaccine and most of the subjects in the study group had Moderna vaccine (N/A: Not Available).
Difference in serum IgG, IgM, and IgA levels between the control and study groups
Our research study showed a non-significant difference in the receptor binding domain (RBD)-specific serum IgG and IgA levels (p=0.40 and p=0.22, respectively) between the control and study groups. There was a significant difference (p=0.0015) in the RBD-specific serum IgM levels between the control and study groups, with the average serum IgM levels found to be much lower in the study group (Figure 1).
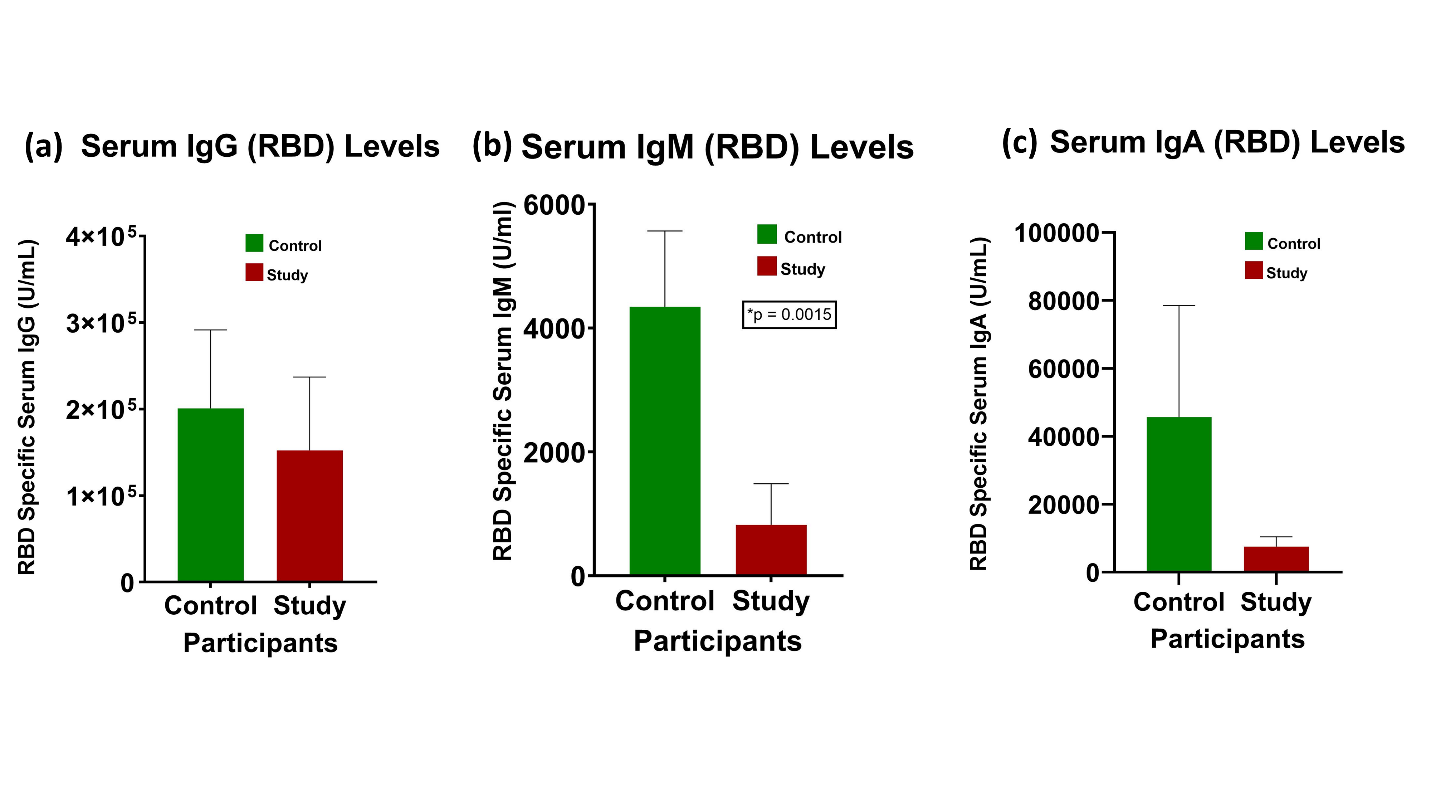
Figure 1.Difference in serum IgG, IgM, and IgA levels between the control and study groups: There was a significant difference in serum IgM levels (p=0.0015) between the two groups. Serum IgG and IgA levels were expressed higher in the control group (Data are normally distributed and presented as Mean ± SEM, Standard Error of Mean).
Percent inhibition from the SARS-CoV-2 neutralizing antibody test
The SARS-CoV-2 neutralizing antibody test’s percent inhibition threshold for determining whether or not the SARS-CoV-2 neutralizing antibodies can be interpreted as functional or not is 30%, with at or higher meaning it can be considered effective at preventing infection. All samples from both the control and study groups exceeded the neutralization threshold (30%), demonstrating effective neutralizing antibodies from both groups (Figure 2).
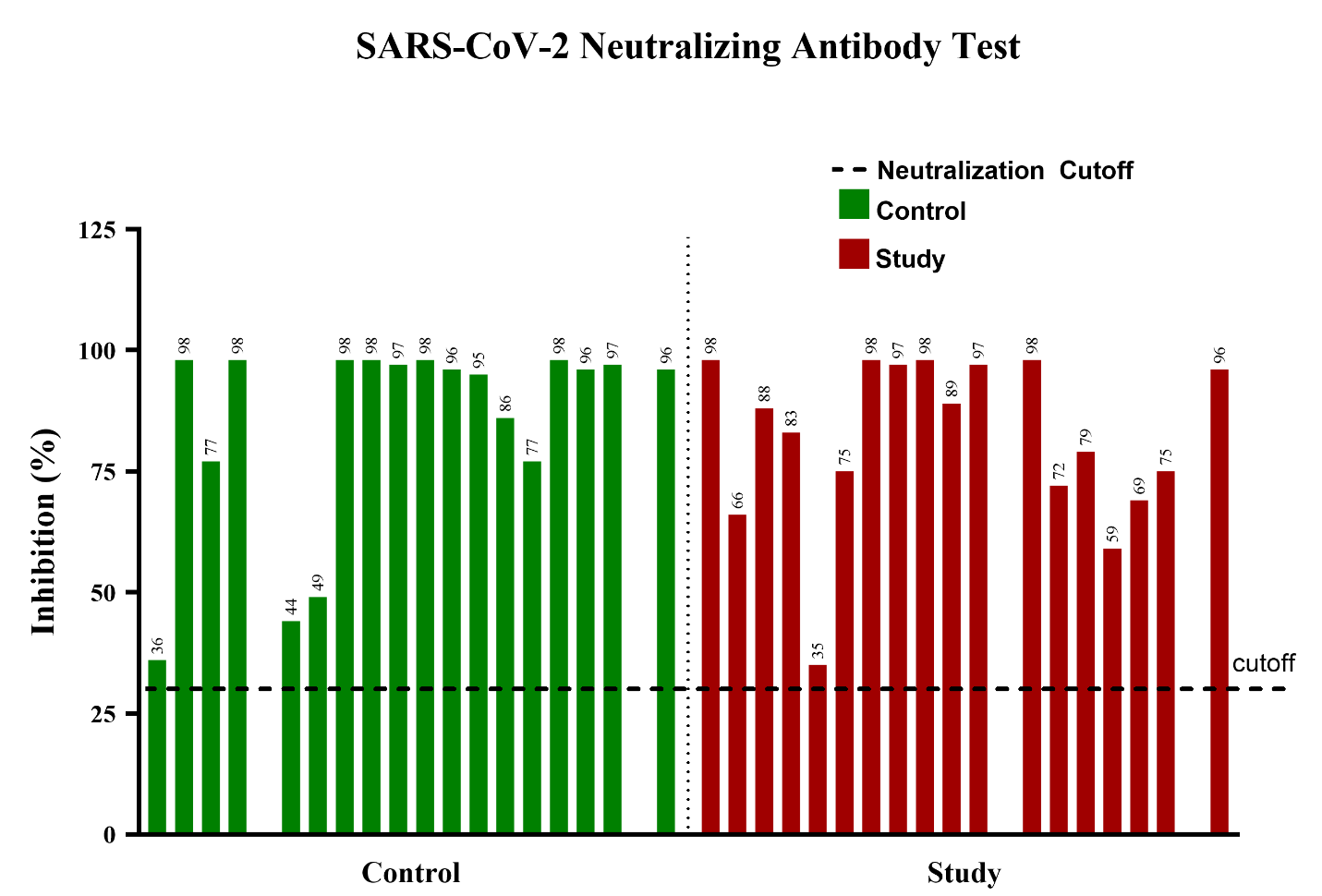
Figure 2. SARS-CoV-2 neutralizing antibody test: All samples from both groups showed effective neutralizing antibodies (Data presented as Mean ± SEM, Standard Error
Discussion
Vaccinating the general population is a crucial public health measure for restricting COVID-19 infections in RA populations. Here, we show the results of the antibody responses (RBD specific serum IgG, IgM, and IgA levels) of control and RA populations to SARS-CoV-2 mRNA-based vaccines (Pfizer or Moderna).
Presently, analyzing IgG levels is a common method for estimating long-term vaccine response, with one study finding a robust correlation between IgG titers and efficacy across seven different vaccines18, 19. The study group’s IgG levels were not significantly different from the control group’s IgG levels, which suggests favorable for the vaccine’s efficacy between rheumatic patients and the general population. IgM and IgA, on the other hand, are more associated with the initial response to infection rather than long-term immunity like IgG. While they play a vital role in early humoral immune response and virus neutralization at mucosal sites, their levels are expected to decrease as time goes on20. Our data follows this trend; an average of six months had elapsed between the booster dose and blood collection, and we found correlating low IgM and IgA levels. Despite this, the disparity between the IgM and IgA levels of the control and study groups spurs interest.
There are few studies addressing the role of IgM in SARS-CoV-2 neutralization. One study showed that there was a strong association between neutralizing antibody response in adults that recovered from a mild case of COVID-19 and the rapid decay of functional antibodies, particularly IgM, over a period of six months following recovery21. Another study found that the virus neutralization capacity rapidly decreases 6 weeks after the onset of symptoms, following a similar trend as anti-RBD IgM. The same study also demonstrated a much stronger connection between neutralizing antibody response and the decay of IgM, rather than IgG or IgA, suggesting that at least part of the neutralization activity is mediated by IgM22.
There is a significant lack of studies during this pandemic regarding IgA responses in individuals who received SARS-CoV-2 vaccination. A recent project conducted by researchers from Australia has reported that convalescent plasma IgA antibodies play an important role in neutralizing the SARS-CoV-2 virus and its variants, despite that neutralizing response being both more heterogeneous and less potent than an IgG response23. Our study has suggested that subjects with autoimmune conditions currently treated with immunosuppressive drugs mount a low serum IgA response after the primary series of SARS-CoV-2 vaccination. Studies and experiments in SARS-CoV-2 vaccines that are able to trigger an IgA response in immunosuppressed individuals are necessary and need to be encouraged24.
The SARS-CoV-2 neutralizing antibody test provides a straightforward way to measure vaccine efficacy. Every single subject in both the control and study groups surpassed the inhibition threshold. Although the neutralizing antibody test provides reliable data, it still has its limitations. Because the surrogate virus used for the test is, by nature, not as infectious as the actual SARS-CoV-2 virus, this may cause inaccurate results. However, the robust results from the antibody test suggest that while this limitation is unlikely to have affected our outcomes, it is still important to consider.
Previous studies have confirmed that patients on RA medications, such as Rituximab and methotrexate, have reduced humoral responses to both influenza and pneumococcal vaccines25. It is not a stretch to suppose that those RA medications may suppress the production of SARS-CoV-2 neutralizing antibodies as well. The researchers from the above study managed to increase the immunogenicity of the seasonal influenza vaccine by temporarily discontinuing RA medication for two weeks post-vaccination without causing a flare-up in RA disease activity25. There is no information available yet on whether this action can be transformed into SARS-CoV-2 mRNA-based vaccines. Our results suggest that patients on immunosuppressive drugs may need alternate vaccination strategies such as additional doses or individual dose modification of mRNA vaccines. Despite the promising outcomes of the post-vaccination neutralizing antibody test and the relatively high IgG levels in the study group, the relatively low IgM and IgA levels are a point of interest. The results warrant further studies to explore the full extent of COVID-19 mRNA vaccine immunogenicity in patients with autoimmune diseases.
Due to immunocompromised patients’ need for alternate vaccination strategies, the effect of high doses versus standard doses of SARS-CoV-2 mRNA-based vaccine immunogenicity is another avenue that needs further research. Efficacy and safety of high-dose vaccination series like influenza and hepatitis B have been studied in immunocompromised individuals and they have proven to be effective26, 27. This data, if confirmed in larger cohorts, could have important clinical implications regarding dosing of vaccination in immunocompromised individuals. Originally, the RA population was excluded from COVID-19 mRNA vaccine trials. Therefore, the efficacy and effectiveness of the SARS-CoV-2 vaccines in this population still need to be established.
Conclusion
Our data on antibody responses, particularly serum IgG, and the neutralizing antibody test indicates that RA subjects with immunosuppressive treatments are able to mount an appreciable immune response after SARS-CoV-2 mRNA vaccination. However, their relatively low serum IgM and IgA levels are notable and suggest further studies into the roles that they play in COVID-19 immunocompetency.
Abbreviations
ACR American College of Rheumatology
ARDS Acute Respiratory Distress Syndrome
CDC Centers for Disease Control and Prevention
COVID-19 Coronavirus disease 2019
CRI Clinical Research Institute
ELISA Enzyme-linked Immunoassay
FDA Food and Drug Administration
Ig Immunoglobulin
IRB Institutional Review Board
MCH Medical Center Hospital
PSO Post-Symptom Onset
RA Rheumatoid Arthritis
RBD Receptor Binding Domain
SARS-CoV-2 Severe Acute Respiratory Syndrome Coronavirus-2
TMB 3, 3’, 5, 5’ tetramethylbenzidine dihydrochloride
TTUHSC Texas Tech University Health Sciences Center
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Authors’ Contributions
The CRI nurses, Ailena Mulkey, Jammie Holland, and Evangelina Santiago obtained the consent forms from all subjects. KG, NJM, AS, SM, and JK drafted the research plan and experiments for the study. KG, NJM, and AS carried out the experiments for the study. KG, NJM, AS, JG, and SM analyzed the data and carried out statistical analysis. KG, NJM, AS, SM, AA, JK, MM, and LPA interpreted the data and contributed to manuscript writing as well as editing. All authors read and approved the final version of the manuscript.
Acknowledgements
We would like to thank Evangelyna Nguyen for helping in the manuscript editing and submission process.
Funding Sources
The authors have no funding sources to declare.
Consent Forms
The consent forms were obtained from all subjects.
References
- Organization WH. COVID-19 weekly epidemiological update, edition 80, 22 February 2022. 2022.
- Alam A, Siddiqui MF, Imam N, et al. Covid-19: current knowledge, disease potential, prevention and clinical advances. Turk J Biol. 2020; 44(3): 121-31.
- Pollard CA, Morran MP, Nestor-Kalinoski AL. The COVID-19 pandemic: a global health crisis. Physiol Genomics. 2020; 52(11): 549-57.
- Gautret P, Million M, Jarrot PA, et al. Natural history of COVID-19 and therapeutic options. Expert Rev Clin Immunol. 2020; 16(12): 1159-84.
- Sharma A, Ahmad Farouk I, Lal SK. COVID-19: A Review on the Novel Coronavirus Disease Evolution, Transmission, Detection, Control and Prevention. Viruses. 2021; 13(2).
- Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020; 215: 108427.
- Tsang HF, Chan LWC, Cho WCS, et al. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev Anti Infect Ther. 2021; 19(7): 877-88.
- Bartoli A, Gabrielli F, Alicandro T, et al. COVID-19 treatment options: a difficult journey between failed attempts and experimental drugs. Intern Emerg Med. 2021; 16(2): 281-308.
- Curtis JR, Johnson SR, Anthony DD, et al. American college of rheumatology guidance for COVIDâ19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis & Rheumatology. 2021; 73(10): e60-e75.
- Bower H, Frisell T, Di Giuseppe D, et al. Impact of the COVID-19 pandemic on morbidity and mortality in patients with inflammatory joint diseases and in the general population: a nationwide Swedish cohort study. Ann Rheum Dis. 2021.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021; 80(10): 1330-8.
- Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018; 15(6): 586-94.
- Shoenfeld Y, Agmon-Levin N. 'ASIA' - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011; 36(1): 4-8.
- Papp KA, Haraoui B, Kumar D, et al. Vaccination Guidelines for Patients With Immune-Mediated Disorders on Immunosuppressive Therapies. J Cutan Med Surg. 2019; 23(1): 50-74.
- Anderson EJ, Rouphael NG, Widge AT, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020; 383(25): 2427-38.
- Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020; 586(7830): 594-9.
- Widge AT, Rouphael NG, Jackson LA, et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N Engl J Med. 2021; 384(1): 80-2.
- Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021; 39(32): 4423-8.
- Ruggiero A, Piubelli C, Calciano L, et al. SARS-CoV-2 vaccination elicits unconventional IgM specific responses in naïve and previously COVID-19-infected individuals. eBioMedicine. 2022; 77: 103888.
- Zhang J, Zhang H, Sun L. Therapeutic antibodies for COVID-19: is a new age of IgM, IgA and bispecific antibodies coming? mAbs. 2022; 14(1): 2031483.
- Harrington WE, Trakhimets O, Andrade DV, et al. Rapid decline of neutralizing antibodies is associated with decay of IgM in adults recovered from mild COVID-19. Cell Reports Medicine. 2021; 2(4): 100253.
- Prévost J, Gasser R, Beaudoin-Bussières G, et al. Cross-Sectional Evaluation of Humoral Responses against SARS-CoV-2 Spike. Cell Reports Medicine. 2020; 1(7): 100126.
- Davis SK, Selva KJ, Lopez E, et al. Heterologous SARS-CoV-2 IgA neutralising antibody responses in convalescent plasma. medRxiv. 2022: 2022.02.06.22270359.
- Wang Z, Lorenzi JCC, Muecksch F, et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Science Translational Medicine. 2021; 13(577): eabf1555.
- Sonani B, Aslam F, Goyal A, et al. COVID-19 vaccination in immunocompromised patients. Clin Rheumatol. 2021; 40(2): 797-8.
- Colmegna I, Useche ML, Rodriguez K, et al. Immunogenicity and safety of high-dose versus standard-dose inactivated influenza vaccine in rheumatoid arthritis patients: a randomised, double-blind, active-comparator trial. The Lancet Rheumatology. 2020; 2(1): e14-e23.
- Anderson EJ, Rouphael NG, Widge AT, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. New England Journal of Medicine. 2020; 383(25): 2427-38.
