An Acute Inflammation with Special Expression of CD11 & CD4 Produces Abscopal Effect by Intratumoral Injection Chemotherapy Drug with Hapten in Animal Model
Baofa Yu, MD1,2,3,4*, Qiang Fu, MS3, Yan Han, MS3, Jian Zhang3, Dong Chen,MD3
1Immuno Oncology Systems, Inc., San Diego, CA 92122, USA
2TaiMei Baofa Cancer hospital, Dongping, Shandong Province, China, 271500
3Jinan Baofa Cancer hospital, Jinan, Shandong Province, China,250000
4Beijing Baofa Cancer Hospital, Beijing, China
Abstract
Aim: To study the immunity reaction in tumor by intratumoral injection with a drug PYM and DNP, where it produces abscopal effect by the expression of immunological genes of tumor on home side (left side) while it brings a similar expression of same genes in tumor on opposite side (right side) of mice.
Method: Prepare each tumor on left side of 6 mice and injected intratumoral with a PYM+DNP and PYM control on day 1 and 7. Two day later of day 1, one of groups mice were inoculated with 0.2ml of H22 cells (105/ml) again on the right underarms as opposite side for controls. On day15, the tumor on bilateral sides were excised for qPCR measurement.
Result: It showed that inflammation with the expression of the CoL1a1, CD4, IL12aÂ, TGFb1Â, Elastin, Elastin, Cox2, CD11b/c, CD8, TNFa in different groups; The inflammation in both side of tumor but these is not only a increasing expression of Collal, CD4, IL12aÂ, TGFb1Â, Elastin, NFKB, Cox2, CD11c, CD8 and TNFa in tumor on home side of mice treated with PYM+DNP but also a similar an increasing expression of same genes in tumor on opposite side of mice which not treated at all. In the control group, it showed that inflammation without an expression of all factors related above immunity genes in both tumor on home treated with PYM only and opposite side of mice which not treated at all.
Conclusion: It indicated that PYM and hapten of DNP can induce an inflammation with stimulation of immunity reaction with the expression of the CoL1a1, CD4, IL12aÂ, TGFb1Â, Elastin, Elastin, Cox2, CD11b/c, CD8, TNFaÂ, which resulted in an abscopale effect. PYM can induce an inflammation but without expression of immuno genes, therefore, hapten is playing an important role with PYM in the special immunity reaction.
Introduction
The abscopal effect is a hypothesis for treating metastatic cancer1. Since 1953, more than half a century, the mechanism of this phenomenon is still unknown. Mole mentioned an overview in the phrase abstract. However, promising new research on the reappearance and immune response to the system is called abscopal effect, which may eventually help us understand the key to metastatic cancer and fine a way of how to reduce and treat the potentially metastasis of cancer. Therefore, there is a growing interest in the systematic use of the therapeutic potential of the systemic response stimulated by radiotherapy.
The present review briefly describes the history of radiotherapy-induced abscopal effects and local irradiation's activation of systemic anti-tumor immune responses2. We must understand the mechanism of abscopal effect in human or animal more deeply. We need to know how to apply the abscopal effect to distant tumor or metastatic cancer in patients through local drugs instead of local radiation, especially for view potential metastasis and control the potential metastasis of tumor cells.
Theoretically, this suggests that tumor necrosis induced by chemical drugs with hapten, it is not natural necrosis of the tumor or ionizing radiation necrosis, can also cause immune responses against tumor cells. It is reported that UMIPIC (ultra-minimum incision personalized intratumoral chemoimmunotherapy therapy, UMIPIC) can induce an excellent anti-tumor immune response in animal models and humans3-6, which provides the possibility of suppressing tumor recurrence and metastasis, such as the abscopal effect. Human tumors are on the rise, which indicates that the human body is tolerant to its tumor antigens. This means that the immune system no longer monitors the tumor antigen, lowering the immune response to tumor cells. That is why, in daily clinical practice, we rarely witness this abscopal effect in clinical practice when the tumor is growing or got chemotherapy or radiation treatment.
As a result of their low immunogenicity, high tolerance, or immune escape, most tumor antigens have difficulty triggering an immune response (immunological tolerance). It has been demonstrated that combining hapten with tumor antigen results in a more potent tumor antigen that can trigger the body's immune response and raise the likelihood of an abscopal effect, which could be critical in regulating the patient's undetectable metastasis. The term "hapten" was coined by Landsteiner and Jacobs7 and is derived from the Greek word "hapten", which means "fasten". A hapten is low molecular weight (<1000 Dalton) chemical substance that must be combined with a carrier molecule to have antigenicity8. Haptenization often occurs when a chemical substance interacts with a protein. After intratumoral injection chemical drug together with hapten, the hapten can easily interact with proteins such as tumor antigens9.
Our published data show that UMPIC provides an ideal intratumoral approach for the chemical de-bulking of advanced pancreatic cancer and advanced hepatocellular carcinoma, and hapten plays a vital role in prolonging the survival rate of patients5-6.
Approved data confirm that UMIPIC can prolong survival time and used hapten of enhance the tumor antigens' immunogenicity with inflammation plus CD4, CD8, CD11 immunostaining positive. Concept of inflammation is red, swell, pain with lymphocytes infiltrate in any tissue included tumor tissues by damaged of virus or bacterial or more, also inflammation is always involved tumor development, which is a chronic inflammation10, in our study, intratumoral injection of drug and hapten can induce an acute inflammation with different expression of immunological genes. Therefore, in order to explore deeply acute inflammation in tumor induced by intratumoral injection of drug and hapten and relationship between the induced an acute inflammation and mechanism of abscopal effect in animal model, we set up animal model with tumor in both side armpit of mice and treat only the one side of tumor, another side of tumor not treated as control, take the both tumor of mice after treatment, analyze and comparison the tumor weight in different group and the expression of different genes related the immunity by qPCR assay.
Materials and Method
Preparation of tumor-bearing mouse
The 8th generation of H22 ascite tumor-bearing of mouse was maintenance for a few weeks at room temperature, mouse’s abdomen was disinfected with 75% ethanol; tumor cell in ascite was collected for extraction on the super clean bench and was diluted with normal saline according to the ratio of 1:2, next, each Babl/C mouse was inoculated with 0.2ml of H22 cells (107/ml) on the underarm of the left foreleg to establish the subcutaneous solid tumor model.
Treatment Method
When the longest diameter of the tumor reached about 4-5mm on left side (home side) of mice as the experiment day 0, the random grouping was performed for injection intratumoral with corresponding drugs combination (Table 1). Next day, an experiment day 1 and tumor on left side of mice were treated by A DNP (2, 4-dinitrophenol) + PYM (Pingyangmycin) and B (PYM) corresponding drugs combination; and at same times, on the right foreleg (opposite side) of mice were inoculated with 0.2ml of H22 cells (105/ml) for observe tumor growth, whether or not abscopal effect happens. The second medication of same injection was performed on the experiment day 7 for left tumor of mice only. The stimulation of inhibition of tumor growth on bilateral sides, especially the opposite side was observed (Table 2, Figure 1.). On the experiment day 15, the 6 mice from each group were executed and the tumor tissues on bilateral sides were excised for qPCR in order to quantitative determination the expression of immunological genes.
Table 1: Experiment dose and grouping
|
Group |
Test sample |
Preparation concentration (mg/ml) |
Medication dose |
Animal number |
|
A: DNP + PYM |
DNP+PYM +H2O2 |
0.8+1.00+20 |
0.8+1.00+20 |
9 |
|
B: PYM |
PYM |
0.8 |
0.8 |
9 |
|
C: Negative |
NS |
9 |
9 |
9 |
DNP:2, 4-dinitrophenol, PYM:Pingyangmycin, total number of model mice: 27 mice
Table 2: Preparation of tumor on both side of mice at different time and treatment design and observation plan
|
Drug combination |
Tumor prepare |
Day 0 |
Day1 |
Day 7 |
Day15 |
|
A: DNP+PYM+H2O2 |
Left side of mice inoculated with H22 cells (105/ml) |
grow to 4-5 mm |
First treatment with A drugs |
Second treatment with A drugs |
Executed and the tumor tissues for qPCR analysis |
|
Right side of mice (no treatment) |
Prepare to inoculate |
Inoculated 0.2ml of H22 cells (105/ml) |
Observe both side tumor growth |
Executed and the tumor tissues |
|
|
B: PYM |
Left side of mice inoculated with H22 cells (105/ml) |
grow to 4-5 mm |
First treatment with B drugs |
Second treatment with B drugs |
Executed and the tumor tissues for qPCR analysis |
|
Right side of mice (no treatment) |
Prepare to inoculate |
Inoculated 0.2ml of H22 cells (105/ml) |
Observe both side tumor growth |
Executed and the tumor tissues for qPCR analysis |
|
|
C: Control |
Left side of mice inoculated with H22 cells (105/ml) |
No medication |
No medication |
No medication |
Executed and the tumor tissues for qPCR analysis |
DNP:2, 4-dinitrophenol, PYM:Pingyangmycin
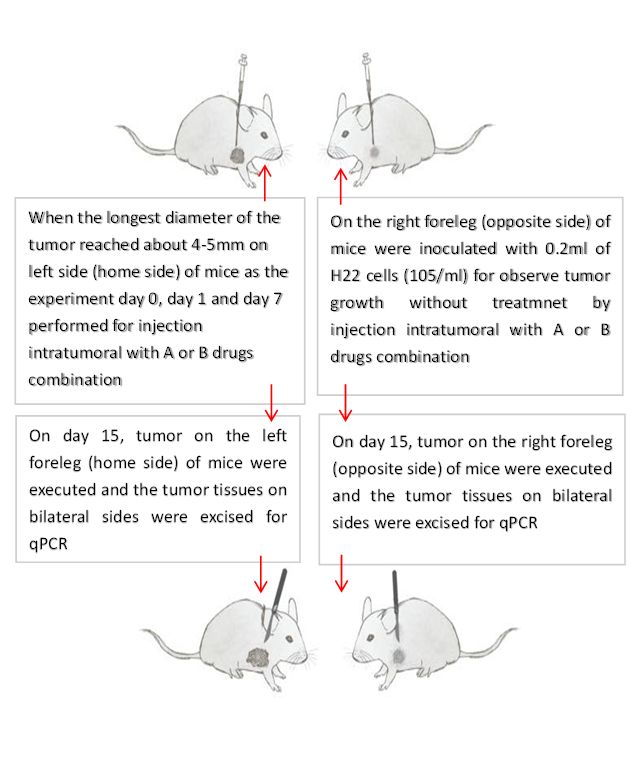
Figure 1: Preparation of tumor on both sides of mice at different time and treatment design for tumor on home side of mouse and observation tumor on opposite side of mouse without treatment.
RNA Isolation and Reverse transcription
The frozen or fresh tumor samples were rapidly transferred to the liquid nitrogen cooled mortar and were ground with continuous add of liquid nitrogen until the tumor tissues turned into powder. The ground powder added to 1.5ml sterilization tube containing 350ul of lysate buffer (lysate Buffer was made by adding 20ul of 50X DTT to every 1ul of Buffer RL). Then the tube was put on the oscillator for oscillation until there was no obvious sediment in the lysate11-12. After filtrate, 600ul of Buffer RWB was added to the RNA Spin Column for the second time. Then mixed solution was put for certification at 12000 rpm for 30 seconds and the filtrated again. The RNA Spin Column was centrifuged at 12000rpm for 2 minutes and then adding in the 1.5ml RNASE Free Column Tube, 50ul of RNASE Free dH2O was added to the RNA Spin Column at the center of the membrane by pipette. After 5 minutes standing at room temperature, the solution was centrifuged for 2 minutes. Then elution of RNA was performed. The eluent of the 1.5 ml RNASE Free Column Tube at the first time could be put back in the RNA Spin Column. After 5 minutes standing at room temperature, 2 minutes concentration at 12000rpm, and elution of RNA, protein electrophoresis was performed10, 11. In the reverse transcription reaction total 4μg of RNA were used, with a maximal volume of 6ul. Reagents from the SuperScript III First Strand Synthesis Super Mix Kit (Invitrogen, San Diego, Ca, USA) were added in the order described in the manual and reaction steps were carried out as stated by the provider. The samples were then kept on -20°C until further use in RT-PCR.
The preparation of the electrophoresis liquid and usage of the PCR apparatus
Related materials were added into 1.5ml EP tube according to the above volume. The 96-well plates were taken out, and 16.8ul of PCR reaction liquid was added to each one. Primers was used for qPCR assay (Table 3). Then, No. 10 primer was added to the first 3 wells, and the remaining primers were added to the wells according to the serial numbers. Next, the plates were covered with micro Amp PCR compatible DNA/RNA/RNASE FREE membrane and placed into 7500 Real Time PCR system (Carlsbad, California 92008, USA). Then the subsequent operations were performed after pressing and entering into the 7500 software v 2.0.6, 20ul of samples were reacted at 95°C for 5s and 60°C for 30s, so it would complete after about 92 minutes11, 12. Conducting qPCR analysis was taken placed by system software.
Table 3: Comparison of Body Weight after Intratumoral Injection (Unit:g)
|
Group Days |
Day 1 |
Day 4 |
Day 6 |
Day 9 |
Day 11 |
Day 13 |
Day 15 |
|
A:DNP +PYM |
27.71±1.80 |
23.11±1.80 |
21.04±1.72 |
21.84±1.72 |
19.17±10.79 |
21.00±8.77 |
24.36±1.28* |
|
B:PYM |
21.10±0.92 |
22.50±0.92 |
21.76±1.22 |
22.56±1.22 |
19.44±1.34 |
18.94±1.34 |
19.24±1.34 |
|
C:Negative |
23.18±1.62 |
24.58±1.62 |
24.37±1.65 |
25.17±1.65 |
26.73±1.26 |
26.23±1.26 |
26.83±1.26* |
Note: “*” Compared with group B p<0.01
Result
Comparison the body weight between the groups, there is not significantly different, but the control group maintains the stable of weight. The weight of mice in PYM + DNP treatment group is lower than that of mice in PYM control group (P<0.01) (Table 4 & Fig. 2). It indicated that PYM + DNP could control tumor growth
Table 4: Comparison the tumor volume after intratumoral injection between left and right side(unit:mm3)
|
Group Days |
Day 1 |
Day 4 |
Day 6 |
Day 9 |
Day 11 |
Day 13 |
Day 15 |
|
A:Left |
128.89±38.12* |
200.00±47.56* |
214.39±56.62* |
222.89±73.35* |
288.11±59.35* |
287.50±79.92* |
307.63±111.27* |
|
B:Left |
179.00±51.11 |
307.72±55.59 |
350.94±88.48 |
407.22±155.72 |
457.28±121.13 |
486.56±104.38* |
684.28±211.99# |
|
C:Left |
245.22±84.43 |
379.06±138.60 |
414.89±114.88 |
499.72±138.35 |
464.61±115.27 |
758.39±190.31 |
1010.61±268.05 |
|
A:Right |
- |
- |
- |
- |
- |
- |
65.88±27.82 |
|
B:Right |
- |
- |
- |
- |
- |
- |
123.11±64.46 |
|
C:Right |
- |
- |
- |
- |
- |
- |
129.11±56.91 |
Note: “*”Compared with the same side of the negative control group p<0.01, “#”Compared with the same side of the negative control group p<0.05
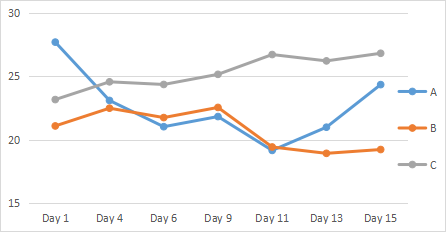
Figure 2: Body weight curve of mice. A:DNP +PYM; B:PYM; C:Negative
There is a significant different between the tumor volume of mice on the left side in each group, the tumor treated with PYM+DNP was smaller than the tumor treated with PYM a lone and that of negative control from day 1 to day 15 (P<0.01). The tumor on right side of mice with late inoculation of tumor cells and no any treatment, the tumor in the PYM +NDP group was smaller than the tumor in PYM alone and negative control group at day15 (P<0.01) (Table 5 & Fig.3), it indicates that the tumor treated PYM+ DNP on the left side of mice could induce the whole body immunity to control the tumor on the right side of mice which is not treated at all.
Table 5: qPCR primers of various factors related to immunity of genes.
|
NFKB Forward |
ATG GCA GAC GAT GAT CCC TAC |
|
NFKB Reverse |
CGG AAT CGA AAT CCC CTC TGT T |
|
Elastin Forward |
TTG CTG ATC CTC TTG CTC AAC |
|
Elastin Reverse |
GCC CCT GGA TAA TAG ACT CCA C |
|
COX2 Forward |
TGC ACT ATG GTT ACA AAA GCT GG |
|
COX2 Reverse |
TCA GGA AGC TCC TTA TTT CCC TT |
|
TNFa Forward |
CTG AAC TTC GGG GTG ATC GG |
|
TNFa Reverse |
GGC TTG TCA CTC GAA TTT TGA GA |
|
TGFb1Â Forward |
GTT CTT CAA TAC GTC AGA CAT TCG |
|
TGFb1Â Reverse |
GTA ACG CCA GGA ATT GTT GCT A |
|
Col1a1 Forward |
CTG GCG GTT CAG GTC CAA T |
|
Col1a1 Reverse |
TTC CAG GCA ATC CAC GAG C |
|
Il12a Forward |
TGC CTT GGT AGC ATC TAT GAG G |
|
Il12a Reverse |
CGC AGA GTC TCG CCA TTA TGA T |
|
CD11c Forward |
CCA AGA CAT CGT GTT CCT GAT T |
|
CD11c Reverse |
ACA GCT TTA ACA AAG TCC AGC A |
|
CD4 Forward |
AGG TGA TGG GAC CTA CCT CTC |
|
CD4 Reverse |
GGG GCC ACC ACT TGA ACT AC |
|
CD8 Forward |
AAG AAA ATG GAC GCC GAA CTT |
|
CD8 Reverse |
AAG CCA TAT AGA CAA CGA AGG TG |
Figure 3: The growth and changes of tumor on home side of mice and the occurrence of tumor on opposite side of mice
By comparing the CTs value in different expression of immunological genes in the two-treatment group with PYM+DNP fro left side of tumor in mice, PYM treatment as control group for left side of tumor in mice (Table 6 & Fig.4). In the experiment, the left side tumor of mice as home side was treated with PYM+DNP while the right side tumor as opposite side was not treated at all, it showed that it is a higher expression of Collal, CD4, IL12aÂ, TGFb1Â, Elastin, NFKB, Cox2, CD11c, CD8 and TNFa in tumor on home side of mice, also there is a similar increasing expression of same factor related with above immunity genes in tumor on opposite side of mice. In the control experiment, the left side tumor of mice as home side was treated with PYM only while right side tumor of mice as opposite side (control side) was not treated at all, it showed that there is not increasing expression of all factors related with above immunity genes in both of home side and opposite tumor of mice, it indicated that PYM alone could not induce an expression of immunity related genes at all, and DNP as hapten is playing an important role in stimulation of immunity reaction with drug of PYM.
Table 6: PCR TC values of left and contralateral tumors at day 15 of experiment
|
Group |
A: Experiment weth PYM and DNP |
B: PYM control |
||
|
Tumor on left side |
Tumor on right ide |
Tumor on left side |
Tumor on right ide |
|
|
Collal |
23.86 |
1.39 |
1.36 |
1.65 |
|
CD4 |
62.6 |
1.94 |
0.58 |
0.71 |
|
1112COA |
58.13 |
1.25 |
0.69 |
0.75 |
|
TGFb1Â |
20.49 |
1.34 |
1.50 |
1.96 |
|
Elastin |
77.24 |
1.19 |
0.23 |
0.34 |
|
NFKB |
21.04 |
0.92 |
0.69 |
0.95 |
|
Cox2 |
54.80 |
2.70 |
0.45 |
0.73 |
|
CD11c |
32.21 |
0.68 |
0.19 |
0.20 |
|
CD8 |
6.33 |
1.04 |
0.51 |
1.00 |
|
TNFa |
164.1 |
3.04 |
2.17 |
6.09 |
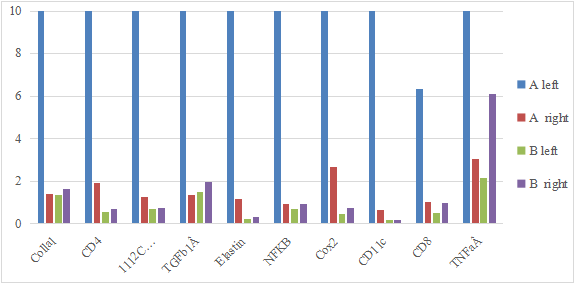
Figure 4.A: The expression of immunological genes from tumor on home side of mice.
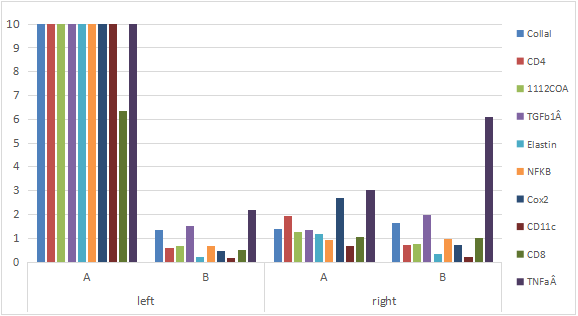
Figure 4: The expression of immunological genes from tumor on opposite side of mice.
By the way, the immunohistochemical staining was performed for CD4, CD8 and CD 11 on some tumor sections and it is confirmed with correlation with the expression of CD4, CD8 and CD11 by qPCR assay (Figure 5), it approved to has effective replacement the immunohistochemical staining by qPCR assay for measure the expression of factors related immune genes, some tumor sections were performed for electronic microscope and it was found the DC cell in tumor tissue after intratumoral injection with drug of PYM and DNP at day 7, DC is an important cell for antigen presentation in the immunological reaction.
Figure 5.A: CD4. The immunohistochemical staining shows the CD4 positive at 100x and 400x in at day 7, it showed CD4 T Cell of special the immune reaction happened after injection intratumoral with drug of PYM and hapten day7, it is confirmed that T cell activation after the intratumoral injection of chemo drug with hapten.
Figure 5.B: CD8. The immunohistochemical staining shows the CD8 positive at 100x and 400x at day 7, it showed expression of CD8 for special the immune reaction happened after injection intratumoral with drug of PYM and hapten day 7, it is confirmed that T cell activation after the intratumoral injection of chemo drug with hapten.
Figure 5.C: CD11. The immunohistochemical staining shows the CD11 positive at 100x and 400x at day 7, it showed CD11 cells activation of special the immune reaction happened after injection intratumoral with drug of PYM and hapten day 7, which it is confirmed that DC activation in tumor after injection chemotheraydrug with hapten.

Figure 5.D: CD11 DC, EM 5μm,it is electronic microscope photo, and it was found that DC cell in tumor tissue after intratumoral injection with drug of PYM and hapten at day7, it is confirmed that DC activation in tumor after injection chemotheraydrug with hapten.
Discussion
Abscopal effect must be related with immunity of patient’s body, the creation of an in situ vaccine library in tumors due to tumor-specific antigens is another attractive factor in the process of intratumoral chemotherapy12-13. In addition, UMIPIC induces vaccine-like effects in tumors and enhances system immunity by adding hapten5, 6, 14,15. When various autologous tumor antigens are released from tumor coagulation, cell death may trigger T cell response and induce effective immunity. These cell deaths are called "good deaths" [16, 17] because immunologic modulators (i.e., small molecule hapten embedded in denatured tumors) promote an in-vivo self-vaccination in the body thus triggering a weak immune response. Our clinical data with UMIPIC and animal studies have shown that the immune response significantly improves after treatment with hapten, especially CD4 + T and B cell immunity response5, 6, 16.
Here, our animal studies of chemotherapy drug plus hapten have further approved the concept of acute inflammation produce an abscopal effect by local delivery chemotherapy drug intratumoral with hapten. Comparison with the weight and tumor volume of mice, it was found that weight is stable for control group, a smaller light weight in PYM+DNP treatment group while tumor volume keeps smaller than the control group, that is good signs for control tumor growth. PYM is a clinically anticancer drug which is used for certain cancer patients17-20, also used for local injection in the past. Today, it is first time for intratumoral injection of PYM with DNP, it may not change the function of PYM, but it may let the PYM to play an important role with DNP separately. In the tumor after injection, PYM still toke a part in the kill tumor cells and made an inflammation there for attracting lymphocytes infiltration which provide a chance for interaction of DNP modify the tumor antigens released from the damage or death of tumor cell killed by PYM to become neu tumor antigens, and a chance for function of DC active in the tumor antigen presenting. It resulted in the high expression of immunological genes of Collal, CD4, IL12aÂ, TGFb1Â, Elastin, NFKB, Cox2, CD11c, CD8 and TNFa in the PYM+DNP treatment group while non expression of same genes in PYM only treatment group; comparison of the expression of genes from tumor on the home site and opposite side of mice which was treated by PYM+DNP, it showed a high expression of immunological genes from tumor on home site of mice while a similar expression of same genes from tumor on opposite of mice, but there is not expression of those genes from tumor on home site and opposite of mice with PYM treated alone. It clearly indicated that chemotherapy drug such PYM must plus hapten such as DNP can work together to make an acute inflammation in the tumor which not only kill tumor cell but also make a cancer vaccination in vitro, in where hapten modify the antigenes like TAA release from damage tumor cell or death tumor cells while chemotherapy drug play an important role of kill tumor cell local effectively for reduce the tumor load which for immunological kill tumor cells easier. After tumor injection with the drug and hapten start up for all of the genes expression, special express of DC11c is for tumor antigen presentation, triggering an immune response specific to these tumor associate antigens (TAA) and warning whole body of immune systems to be ready for fighting, it is an immune storm, the different gene expression may have a different function, some is for T cell function like CD4 and CD8, IL12aÂ, TGFb1Â, TNFaÂ, some may be for B cell function, antologous antibodies of TAA was measured to be higher than those before treatment in cancer patient’s samples, like P53 and P16, Zeta genes which will be reported later.
Conclusion
Chronic inflammation is a bad thing for tumor development, drug induced acute inflammation may be a good thing for control tumor, chemotherapy drug of PYM +DNP intratumoral injection can make an acute inflammation in tumor, meanwhile it provide an opportunity start up an immunity reaction of mice with DNP modify TAA to become neu tumor associate antigens, it resulted in the expression of immunological genes from tumor on home site of mice and also trig and provoke a similar expression of immunological genes from tumor on opposite site of mice and the special immunity reaction in whole body of mice. It has further confirmed the abscopal effect must be related with acute inflammation in tumor and special immunological reaction in body which including expression of Collal, CD4, IL12aÂ, TGFb1Â, Elastin, NFKB, Cox2, CD11c, CD8 and TNFaÂ, and make it a special acute inflammation different from traditional inflammation and become a biological manufacture for immunological factor or cancer vaccine production. Special express of DC11c is for tumor antigen presentation, triggering an immune response specific to these tumor associate antigens (TAA) and warning whole body of immune systems to be ready like an immune storm for fighting.
In fact, it offers a novel eclectic approach for treating cancer with cut off tumor load dramatically by intratumoral injection with chemotherapy drug while in tumor the DNP modify with TAA in vivo into neuantigens to play a role to induce abscopal effect to against micro metastasis in the body of cancer patients.
References
- Whole-body irradiation; radiobiology or medicine? Mole RH. Br J Radiol. 1953; 26: 234–241.
- Epstein AL, Chen FM, Taylor CR. A novel method for the detection of necrotic lesions in human cancers. Cancer research.1988; 48(20): 5842-8. Epub 1988/10/15. PubMed PMID: 3048650.
- Goldberg EP, Hadba AR, Almond BA, et al. Intratumoral cancer chemotherapy and immunotherapy: opportunities for nonsystemic preoperative drug delivery. The Journal of pharmacy and pharmacology. 2002; 54(2): 159-80. Epub 2002/02/19. PubMed PMID: 11848280.
- Vogl TJ, Wissniowski TT, Naguib NN, et al. Activating tumor-specific T lymphocytes after laser-induced thermotherapy in patients with colorectal liver metastases. Cancer immunology, immunotherapy:CII. 2009; 58(10): 1557-63. Epub 2009/02/03. doi: 10.1007/s00262-009-0663-1. PubMed PMID: 19184001.
- Jing P, Li J, Gao F, et al. Use of Hapten Combined Cytotoxic Drugs for Enhancing Therapeutic Effect in Advanced Stages of Pancreatic Cancer. Journal of Liver Research, Disorders & Therapy. 2015; 1(3): 00013.
- Yu B, Lu Y, Gao FJ, et al. Hapten-enhanced therapeutic effect in advanced stages of lung cancer by ultra-minimum incision personalized intratumoral chemoimmunotherapy therapy. Jopurnal of Hepatocellular carcinomars. 2015; 2: 1-11.
- Landsteiner K, Jacobs J. Studies on the Sensitization of Animals with Simple Chemical Compounds. Ii. The Journal of experimental medicine. 1936; 164(4): 625-39. Epub 1936/09/30. PubMed PMID: 19870557; PubMed Central PMCID: PMC2133443.
- Chipinda I, Hettick JM, Siegel PD. Haptenation: chemical reactivity and protein binding. J Allergy (Cairo). 2011; 2011: 839682. doi: 10.1155/2011/839682. Epub 2011 Jun 30.
- Smith JCM and Hotchkiss Allergic Contact Dermatitis: Chemical and Metabolic Mechanisms, Taylor and Francis, London, UK, 2001.
- Takuji Tanaka. Introduction for inflammation and cancer. Semin Immunopathol. 2013 Mar; 35(2): 121-2. doi: 10.1007/s00281-012-0360-6. Epub 2013 Jan 9.
- Kuang J, Yan X, Genders AJ, et al. An overview of technical considerations when using quantitative real-time PCR analysis of gene expression in human exercise research. PLOS ONE13(5): e0196438. PLOS ONE | ttps://doi.org/10.1371/journal.pone.0196438 May 10, 2018.
- Funato T, Takeda M, Funato T, et al. Quality assurance of quantitative PCR with real-time reverse transcription polymerase chain reaction method. 2006; 54(9): 910-7. Sep. Rinsho Byori. 2006.PMID: 17063872
- Almond BA, Cuevas BJ, Enriquez I, et al. Nano-Mesosphere Drug Carriers for Localized Cancer Chemotherapy. TechConnect Briefs, 2006; May 7, (2): 1-14.
- Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. Dec 19; 2005; 202(12): 1691-701.
- Qiong J, Yu B. Slow intra-tumor release of drugs on B16 melanoma in mice. J Shandong Univ 45: 988-992,
- Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003; 15; 170(10): 4905-13.
- Ming Y, Ying-xin Z. Expressing cytokines mRNA induced by B7 gene Jurkat cells by cytarabine. J Biochem Pharmaceutic 2009; 30: 6-13.
- Wu HW, Wang X, Zheng JW, et al. Treatment of deep-seated facial microcystic lymphatic malformations with intralesional injection of pingyangmycin. Medicine (Baltimore), 201; Sep; 95(37).
- Pingyangmycin inhibits glycosaminoglycan sulphation in both cancer cells and tumour tissues. J Cell Mol Med. 2020; 24(6): 3419-3430. doi: 10.1111/jcmm.15017.Epub 2020 Feb 18.
- Yuan Wl, Wang Xk, Xue L, et al. Clinical evaluation and animal experimental study of different mass concentrations of pingyangmycin in the local injection treatment of lip venous malformation. Ann Transl Med Jun; 2021; 9(11): 929.
