Implementation of Immunometabolism into Curricula, Scientific Societies, and Immunological Routine Diagnostics
Marc Roder1 and Sascha Kahlfuss1,2,3,4*
1Institute of Molecular and Clinical Immunology, Medical Faculty, Otto-von-Guericke University Magdeburg, Magdeburg, Germany.
2Institute of Medical Microbiology and Hospital Hygiene, Medical Faculty, Otto-von-Guericke University Magdeburg, Magdeburg, Germany.
3Health Campus Immunology, Infectiology and Inflammation (GCI3), Medical Faculty, Otto-von-Guericke University Magdeburg, Magdeburg, Germany.
4 Center for Health and Medical Prevention (CHaMP), Otto-von-Guericke-University, Magdeburg, Germany.
Abstract
The energy metabolism was demonstrated to directly modulate immune cell function and thereby physiological and detrimental immune responses. In addition, the field of immunometabolism is vastly growing. However, yet there remain fundamental scientific questions in the field, which require the organization of national and international networks as well as the implementation of immunometabolism into curricula, scientific societies, and immunological routine diagnostics to hold the promise of personalized medicine to our patients within the next decade.
Introduction
In very recent years, a large body of significant studies has investigated the energy metabolism of innate and adaptive immune cells in immunity to tumors and pathogens as well as in autoimmune diseases1-3. Through these pioneering studies, it has become clear that immune cells are highly dependent on an effective energy metabolism to fulfill their requirements for proliferation, differentiation, and cell type-specific effector functions. Yet, it is established that different immune cells use distinct types of metabolic pathways. In this context, the type of energy metabolism used by immune cells is significantly influenced by their developmental and differentiation stage2, 4, 5. Recently, it was for example demonstrated that T helper (Th)17 cells, which are critical for immunity to extracellular bacteria and fungi but also contribute to autoimmunity, are composed of a unique ultrastructure with fused mitochondria and tight christae, making them highly dependent on intact mitochondrial architecture and metabolic pathways6. Independent of such intrinsic energy programs, metabolic pathways and the effector functions of immune cells are also influenced by an extrinsic metabolic environment within the organ. The extrinsic metabolic microenvironment can even significantly vary within the very same organ, depending on whether the organs are affected by a disease or not7-10.
An important step in our recent understanding was learning that immune receptor activation cumulates in the induction of specific metabolic genes in immune cells. For instance, antigen specific activation of the T cell receptor (TCR) controls the expression of genes that encode for glycolytic enzymes1. Early after T cell activation it is thus a switch to glycolysis that ensures the generation of sufficient building blocks for consecutive clonal expansion of hundreds and thousands of effector T cells directed towards one single antigen. Another important milestone in the field was the discovery that the metabolism of immune cells, inversely, can directly affect their development, differentiation, and effector function through the control of cellular fitness and survival, by maintaining cell homeostasis especially in metabolically challenging microenvironments during diseases, or even through epigenetic gene regulation mechanisms11-13.
Understanding Immunometabolism for Diagnostics and Therapy
Although we have developed a relatively detailed understanding of how metabolic pathways and immune cell effector functions are interconnected, there are still yet open questions: i) How does the metabolic microenvironment affect the energy metabolism and effector function of immune cells? To answer this question, a detailed characterization of metabolic microenvironments within organs and compartments in health and disease is needed. ii) While many studies have focused on adaptive immune cells so far, there is a lack in our understanding of how myeloid cells and their plasticity is shaped by their energy metabolism or by the distinct metabolic microenvironment they operate in. For example, mast cells have a unique proteome and molecular machinery that ensures vesicle formation, storage and release. However, it is almost completely unknown how metabolic pathways contribute to build up the prerequisite that ensures mast cell effector function. iii) Despite our understanding of which type of energy metabolism is favored by distinct immune cell populations, we are just at the beginning in engineering pipelines that allow us to use this knowledge to improve immunotherapies, for example to enhance the fitness of chimeric antigen receptor (CAR) T cells.
For CAR T cell therapy, T cells are extracted from patients and genetically engineered to express CARs that consist of an extra-cellular antigen binding domain connected with an intracellular signalling domain14. This enables CAR T cells to bind and kill tumour cells when reinfused to patients. What looks in theory like a universal form of treatment for malignant diseases is in reality confronted with varying success also due to challenges in maintaining metabolic fitness of CAR T cells during manufacturing or after CAR T cells have been administered to the patient15, 16, 17. Here, further studies are needed especially with the increasing number of diseases that can be efficiently targeted by CAR T cells. In this context, CAR T cells were recently reported to evoke ‘immunological’ remission of patients suffering from systemic Lupus erythematosus (SLE) patients18, 19. Furthermore, a better understanding of metabolic changes in innate and adaptive immune cells during systemic pathological conditions in the human body could further help in diagnosing diseases or evaluating treatment efficiency. In addition, the spectrum of diseases that will be treatable with CAR T cells in future is likely to grow exponentially within the next decade and we are just at the beginning20.
To date, scientific societies have not significantly implemented immunometabolism in their agenda. This is in stark contrast to the fact that metabolic pathways in immune cells were demonstrated to directly impact the function of several immune cell types and, thereby, to control outcomes of various diseases. These studies establish the metabolism of immune cells beyond a simple epiphenomenon of their functional state.
Conclusion
As the field of immunometabolism is a young research area, the establishment of an independent working group ‘Immunometabolism’ within scientific societies such as for instance the German Society of Immunology (DGfI) would be a unique opportunity to combine both, the support of a young community as well as the foundation of a working group that is needed.
In addition, most of the curricula for students of human and veterinary medicine as well as of several biological or engineering disciplines do not have implemented courses on immunometabolism yet. This likely will change in the near future as meanwhile many (but too less) universities have established professorships in immunology with a particular focus on immunometabolism. Implementing immunometabolism within scientific societies, curricula, diagnostics, and research is more than an academic exercise as a detailed understanding of the energy metabolism and how it affects immune cell function will be crucial for tailor-made immunotherapies such as CAR T cell technologies. So far, there are a few examples of immunometabolism research initiates, organizations and scientific networks such as the “immunometabolism group” in Sydney at the University of New South Wales or the “IMMUNOMET EU” working group in Europe (Figure 1). We need more of them and have to switch gears and implement immunometabolism within scientific societies, curricula, and diagnostics. Otherwise, we will be lacking the community, educated people, the technology, and further vibrant research studies that guarantee the implementation of next generation immunotherapies for our patients relatively soon.
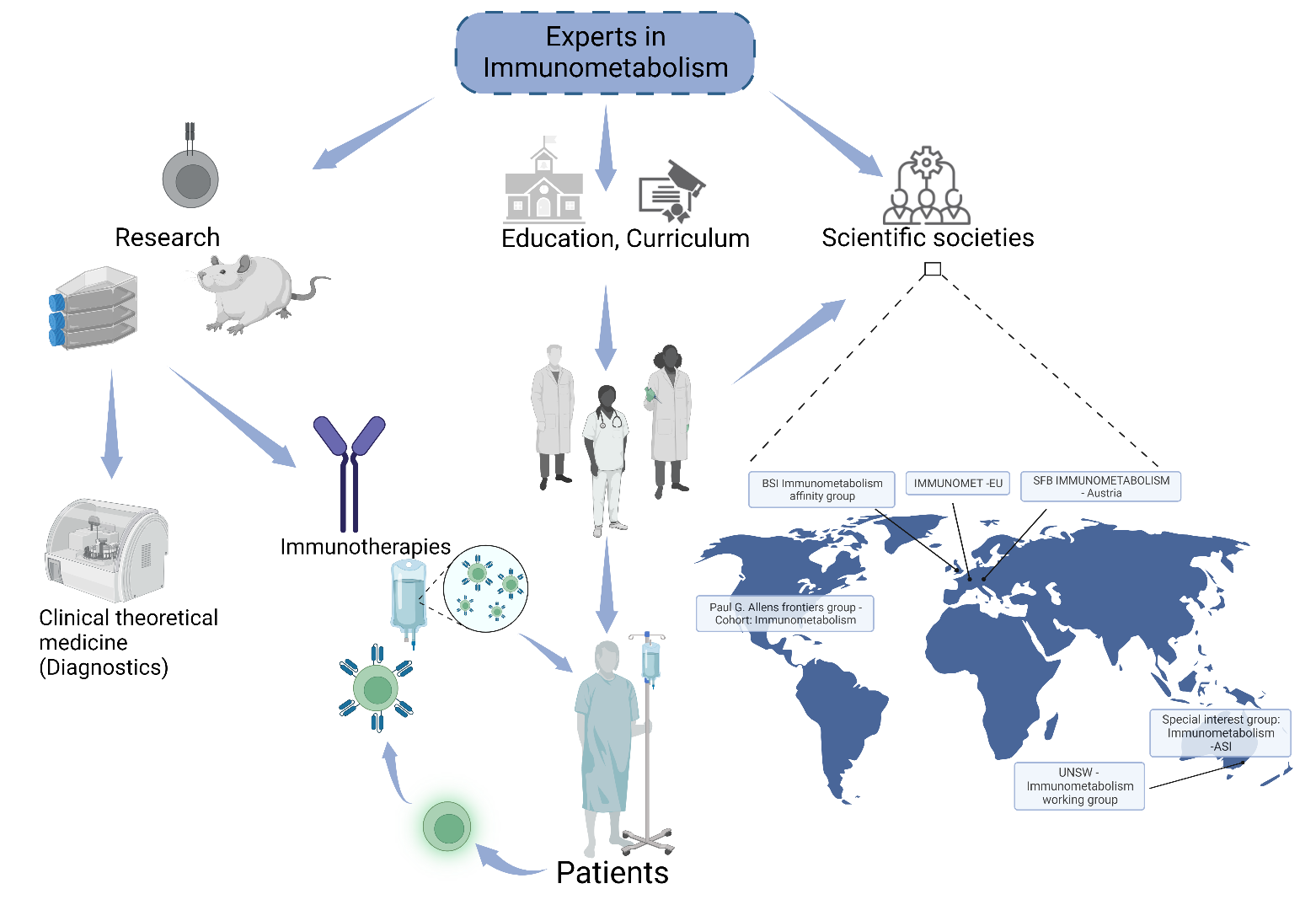
Figure 1: Implementation of immunometabolism into curricula, scientific societies, and immunological routine diagnostics: Cartography shows examples of immunometabolism research initiatives, organizations and networks across the world. Created using www.biorender.com
Author contribution
M.R. and S. K. drafted, wrote, edited, corrected and illustrated the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – 361210922/GRK2408 – (Project 12) to S.K.
References
- Buck MD, Sowell RT, Kaech SM, et al. Metabolic Instruction of Immunity. Cell. 2017 May 4;169(4):570-586. doi: 10.1016/j.cell.2017.04.004. PMID: 28475890; PMCID: PMC5648021.
- Geltink RIK, Kyle RL, Pearce EL. Unraveling the Complex Interplay Between T Cell Metabolism and Function. Annu Rev Immunol. 2018 Apr 26; 36: 461-488. doi: 10.1146/annurev-immunol-042617-053019. PMID: 29677474; PMCID: PMC6323527.
- Pearce EL. Metabolism as a driver of immunity. Nat Rev Immunol. 2021 Oct; 21(10): 618-619. doi: 10.1038/s41577-021-00601-3. PMID: 34580450.
- Michalek RD, Gerriets VA, Jacobs SR, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011 Mar 15; 186(6): 3299-303. doi: 10.4049/jimmunol.1003613. Epub 2011 Feb 11. PMID: 21317389; PMCID: PMC3198034.
- Puleston DJ, Baixauli F, Sanin DE, et al. Polyamine metabolism is a central determinant of helper T cell lineage fidelity. Cell. 2021 Aug 5; 184(16): 4186-4202.e20. doi: 10.1016/j.cell.2021.06.007. Epub 2021 Jul 2. PMID: 34216540; PMCID: PMC8358979.
- Baixauli F, Piletic K, Puleston DJ, et al. An LKB1-mitochondria axis controls TH17 effector function. Nature. 2022 Oct;610(7932):555-561. doi: 10.1038/s41586-022-05264-1. Epub 2022 Sep 28. PMID: 36171294.
- Hortová-Kohoutková M, LázniÄková P, FriÄ J. How immune-cell fate and function are determined by metabolic pathway choice: The bioenergetics underlying the immune response. Bioessays. 2021 Feb; 43(2): e2000067. doi: 10.1002/bies.202000067. Epub 2020 Nov 16. PMID: 33191545.
- Alwarawrah Y, Kiernan K, MacIver NJ. Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front Immunol. 2018 May 16; 9: 1055. doi: 10.3389/fimmu.2018.01055. PMID: 29868016; PMCID: PMC5968375.
- Caputa G, Castoldi A, Pearce EJ. Metabolic adaptations of tissue-resident immune cells. Nat Immunol. 2019 Jul; 20(7): 793-801. doi: 10.1038/s41590-019-0407-0. Epub 2019 Jun 18. PMID: 31213715.
- Karmaus PWF, Chen X, Lim SA, et al. Metabolic heterogeneity underlies reciprocal fates of TH17 cell stemness and plasticity. Nature. 2019 Jan; 565(7737): 101-105. doi: 10.1038/s41586-018-0806-7. Epub 2018 Dec 19. PMID: 30568299; PMCID: PMC6420879.
- Almeida L, Lochner M, Berod L, et al. Metabolic pathways in T cell activation and lineage differentiation. Semin Immunol. 2016 Oct; 28(5): 514-524. doi: 10.1016/j.smim.2016.10.009. Epub 2016 Nov 4. PMID: 27825556.
- Franco F, Jaccard A, Romero P, et al. Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab. 2020 Oct; 2(10): 1001-1012. doi: 10.1038/s42255-020-00280-9. Epub 2020 Sep 21. PMID: 32958939.
- Hochrein SM, Wu H, Eckstein M, et al. The glucose transporter GLUT3 controls T helper 17 cell responses through glycolytic-epigenetic reprogramming. Cell Metab. 2022 Apr 5; 34(4): 516-532.e11. doi: 10.1016/j.cmet.2022.02.015. Epub 2022 Mar 21. PMID: 35316657; PMCID: PMC9019065.
- Zhang C, Liu J, Zhong JF, et al. Engineering CAR-T cells. Biomark Res. 2017 Jun 24; 5: 22. doi: 10.1186/s40364-017-0102-y. PMID: 28652918; PMCID: PMC5482931.
- Kawalekar OU, O'Connor RS, Fraietta JA, et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016 Feb 16; 44(2): 380-90. doi: 10.1016/j.immuni.2016.01.021. Erratum in: Immunity. 2016 Mar 15; 4 4(3): 712. Snyder, Nathaniel [corrected to Snyder, Nathaniel W]; Blair, Ian [corrected to Blair, Ian A]. Erratum in: Immunity. 2016 Mar 15;44(3):712. PMID: 26885860.
- Salter AI, Ivey RG, Kennedy JJ, et al. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci Signal. 2018 Aug 21; 11(544): eaat6753. doi: 10.1126/scisignal.aat6753. PMID: 30131370; PMCID: PMC6186424.
- Böttcher-Loschinski R, Rial Saborido J, Böttcher M, et al. Lipotoxicity as a Barrier for T Cell-Based Therapies. Biomolecules. 2022 Aug 25; 12(9): 1182. doi: 10.3390/biom12091182. PMID: 36139021; PMCID: PMC9496045.
- Mougiakakos D, Krönke G, Völkl S, et al. CD19-Targeted CAR T Cells in Refractory Systemic Lupus Erythematosus. N Engl J Med. 2021 Aug 5; 385(6): 567-569. doi: 10.1056/NEJMc2107725. PMID: 34347960.
- Mackensen A, Müller F, Mougiakakos D, et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med. 2022 Oct; 28(10): 2124-2132. doi: 10.1038/s41591-022-02017-5. Epub 2022 Sep 15. PMID: 36109639.
- Lercher A, Baazim H, Bergthaler A. Systemic Immunometabolism: Challenges and Opportunities. 2020 Sep 15; 53(3): 496-509. doi: 10.1016/j.immuni.2020.08.012. PMID: 32937151; PMCID: PMC7491485.
