Plasmacyte Heterogeneity of Lame Ducklings
Paul F. Cotter
Cotter Laboratory, Arlington, MA, 02476, USA
Abstract
The aim is to describe the array of plasmacytes (PC), cells known as the source of antibody, occurring in the bone marrow (BM) of lame ducklings. The method is by a light microscopic examination of touch preparation slides made from femur samples and stained with Wright-Giemsa. Samples were obtained on site at commercial farms where slide preparations were made; reducing the possibility that observations are technical artifacts.
The results: indicate that PC occur in a multitude of sizes, shapes, nuclear/cytoplasmic (N/C) ratios, ploidy, and nuclear and cytoplasmic conditions. Normal PC are illustrated first, followed by atypical forms. Some PC are presented in the context of neighboring BM cells of the granulocyte, erythrocyte, and reticulum cell (histiocyte) series.
More than 100 Mott-type PC were measured in a single sample from a 13-day lame duck; and several distinct forms were identified. Size, as measured by their longest axis, varied from 6.1 to 28 μm and it appears to be normally distributed. Moreover, N/C ratios were distributed across a three-fold range (0.3 – 0.9) indicating Mott phenotypes can occur at multiple developmental stages. Motts differed in Russell Body (RB) size, and nuclear condition. A novel Mott type, “orb” form, with partially lysed nuclei is also described.
PC were often found in association with giant granulated histiocytes (ggh) and non-granulated giant histiocytes (gh). Other atypical forms are “hand-mirror” PC, trinucleate and binucleate PC resembling cells seen in multiple myeloma (MM) and lymphomas.
Collectively these PC variants constitute “reactive plasmatosis” (RP) likely arising as a consequence of the presence of various bacteria including Streptococcus and E. coli.
The conclusions: It is demonstrated that RP as occurs in lame ducklings suffering from bacterial infections, provides a unique theater for the study of PC variability. Atypical PC, some resembling neoplastic types, were common in RP BM. The significance of the study relates to the importance of PC in disease and immunity; therefore, these observations should interest those who specialize in these areas. They expand the knowledge of avian plasmacyte morphology.
Introduction
Mature plasmacytes (PC) the source of antibody and members of the lymphocyte series, are derived by maturation of B-cells. Development of B cells occurs in the specialized microenvironment of the bursa of Fabricius. There immunoglobulin rearrangement events, intrachromosomal gene conversion, a mechanism distinct from mammals, and maturation of the pre-plasmacyte B-cell occurs1.
When stained with Romanowsky dyes, the condensed chromatin of the PC eccentric nucleus appears in a “clock-face” or “cart-wheel” configuration. An extranuclear clear area (Hof) the location of the Golgi apparatus, where secretory products are assembled, is also a prominent feature. Mature cells have a nuclear/cytoplasmic ratio (N/C) of < 0.5. Although rare in the normal circulation, plasmacytes comprise a small percentage of normal BM cells (< 1%) where they are scattered among granulocyte and erythrocyte progenitors2.
In response to antigenic stimulation the situation becomes more complex. Peripheral PC multiply, some migrate to the BM, atypical cells occur, and some can be detected in the circulation3-6. At this point PC recognition becomes more challenging as reactive lymphocytes and early developmental stages comingle with mature and atypical types.
In humans, PC dyscrasias range from benign processes to aggressive malignancies. PC morphology parallels the diversity of symptomology. In multiple myeloma (MM) a myriad of genetic events including chromosomal translocations, trisomy and other rearrangements of Ig genes result in expansion of PC clones7. In RP overproduction of inflammatory cytokines results in expansion of the PC repertoire that may mimic malignancy8.
In 6 wk ducklings, atypical PC with distinct cytoplasmic features, Mott cells, were seen in BM accompanied by bacteria4,5. These cells stand out because the accumulated secretions dilate the endoplasmic reticulum giving a “bunch of grapes” appearance. However additional PC variants have been described in MM and other diseases9. Here, it is the aim to expand the knowledge of avian PC morphologic variation by describing cells of the BM of young ducklings afflicted by lameness; a condition favoring development of RP. Where possible PC will be presented in the context of neighboring cells of the granulocyte and reticulum cell (histiocyte) series. Cells displaying classic PC features will be presented first followed by examples of atypical PC. The study of the mechanisms regulating PC proliferation, the development of atypical forms, and their death should aid the understanding of basic biology of immunity.
Materials and Methods
Ducklings and welfare
Ducklings ranging in age from 7 to 28 days were examined at commercial farms. All were grown on floor pens covered with wood shavings. Six lame ducklings were identified by experienced field service personnel who removed them from further harm and prepared the sample slides on site. All animal procedures follow the “Guide for the Care and Use of Laboratory Animals” published by Institute for Laboratory Animal Research (ILAR Guide, 1996) (http://www.nap.edu/openbook.php?record_id=5140).
Flock welfare is monitored under the Maple Leaf Farms Trident Stewardship Program for Duck Well Being and procedures were reviewed by a PAACO certified auditor and licensed Veterinarian.
Sample collection
Touch preparation slides were made at each farm site from femur BM. Specimens were spread across clean glass microscope slides and immediately air dried. Later, slides were immersed in 95% ethanol and postfixed for 10 to 15 min. Slides were stained by Wright’s method followed by a brief exposure to Giemsa using an in-house procedure.
Light Microscopy and photomicrographs
Olympus CX-41(Olympus America, Center Valley, PA 18034-0610) equipped with Plan N 40x, 0.65 numerical aperture dry, and Plan N, 1.25 numerical aperture 100x oil objectives. All images were captured at 100x with an Infinity-2 1.4-megapixel charge-coupled device Universal Serial Bus 2.0 Camera and processed with Infinity Analyze software (Release 6.5) (Lumenera, Inc., Ottawa, ON, Canada).
Statistics
Frequency distribution graphics were done using Minitab Statistical Software (Release 17 for Windows, Minitab Inc., State College, PA).
Results
In order to conserve space figure legends are simplified; detailed descriptions of individual cells appear in the text.
Normal plasmacytes
When present in the circulation or at increased frequency in BM up-regulation of the immune system is indicated. A classic or “normal” PC has certain recognized features shown in Figure 1; additional examples are in avian hematology literature3-5,10. Panel A, four oval cells display a “clock-face” chromatin configuration with N/C ratios (0.3 or 0.4) typical of classic or “normal” PC. The deeper cytoplasmic hue of cells 1 and 2 possibly suggests they are less mature than cells 3 and 4 whose cytoplasm is of a slightly lighter shade. Cell 5 types are mature classic heterophils (HC)3-6 and cell 6, containing both primary and specific granules, is a HC metamyelocyte (mtm)2. A sequence of proplasmacytes (pPC) at various developmental stages is in Panel B. Cell 1, with deeply stained cytoplasm, is an early stage (N/C 0.63) and is slightly distorted due to pressures from neighboring cells. Cell 2, the largest (pPC; N/C 0.75) has early cytoplasm also. The oval shape of cell 4 is a characteristic of mature PC (N/C 0.41); its large irregular cytoplasmic vacuoles suggest it could ultimately develop into a Mott cell (see below). The chromatin of cells 1 and 2 is more open, an indication of an early stage cell9
.it contrasts with more condensed chromatin of cells 3 and 4. Collectively the cells of Figure 1 suggest the range of morphology expected of “normal” pPC/PC types; atypical cells are described below.
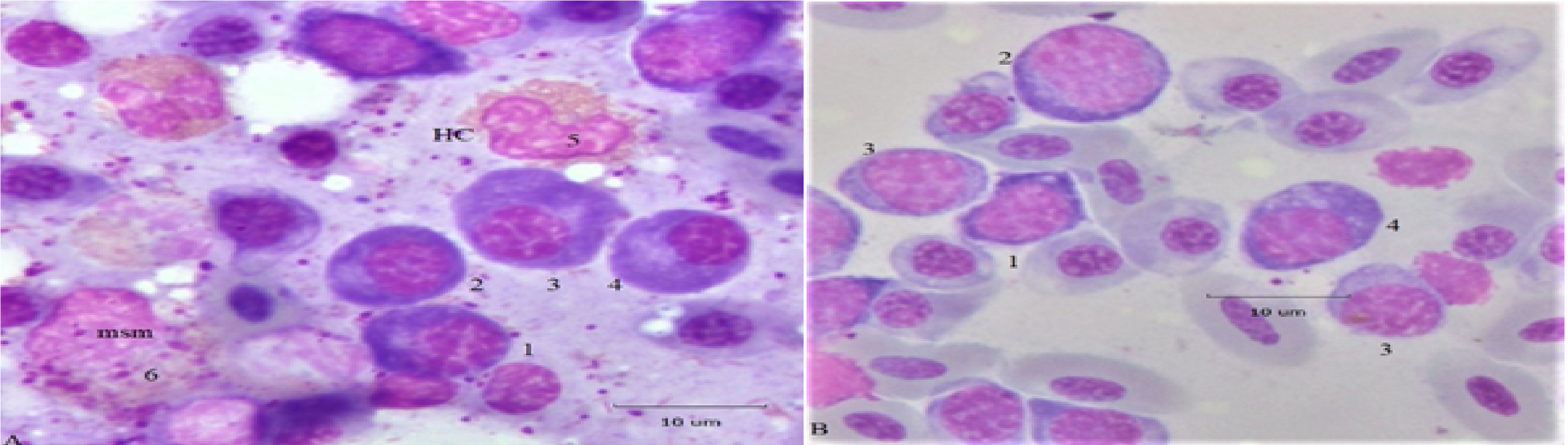
Figure 1: Normal and developmental plasmacytes. Panel A, four PC displaying classic nuclear and cytoplasmic characteristics are located near heterophils and a meso/metamyelocyte (msm). Panel B, a sequence of proplasmacytes (pPC) at various early (1) and later (2, 3, 4) developmental stages. Additional descriptions are in the text.
PC associations with other series
The association of proplasmacytes with giant granulated histiocytes (ggh) is displayed in Figure 2. Cell 1, a small lymphocyte (Ls) is partially engulphed by the cytoplasm of a ggh. Cell 2 is a plasmacytoid cell, with partially condensed chromatin and a perinuclear clear area (Hof); its distended endoplasmic reticulum is seen as unstained cytoplasm. Cells 3, 4, and 5 are large pPC with open chromatin and N/C ratios of (0.7, 0.6, 0.4, respectively). The clear Hof of each cell indicates a capacity for secretion. In contrast, their violet cytoplasm suggests they retain a primitive state11. Both the nucleus and cytoplasm of cell 5 contain conspicuous vacuoles suggesting it may morph into a Mott type PC. Cell 6, a pPC with condensed chromatin, is at the prophase stage. The nuclei of both ggh are swollen and partially lysed. The topmost ggh has phagocytosed an erythroplastid (ep) an anuclear RBC fragment12. Weakly stained HC (cell 7) display varying degrees of necrosis. Background RBC are a mixture of mature and polychromatic cells (pRBC). Similar PC-histiocyte associations, called “satellite formations”, are known in multiple myeloma13 but here their relation is more likely nurturing via cytokines, a role attributed to “stromal cells”14.
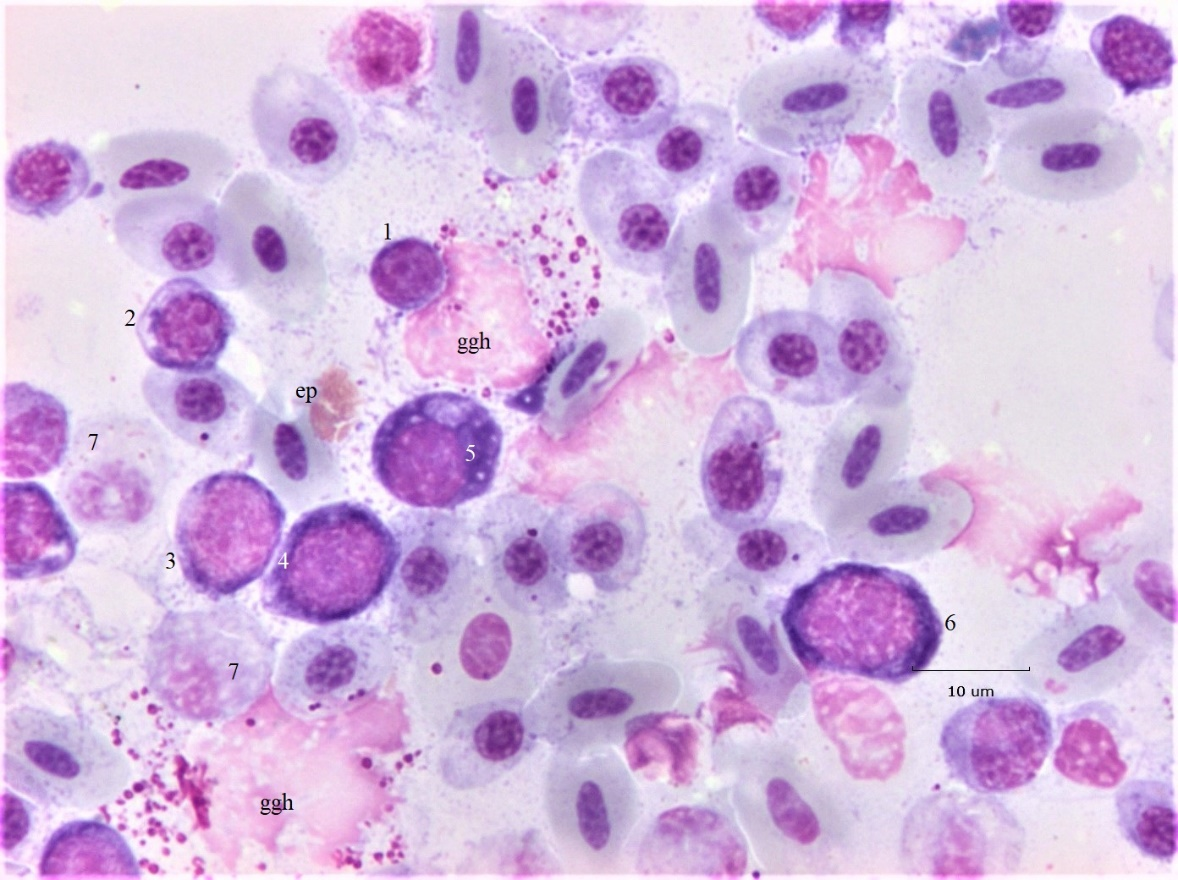
Figure 2: Bone marrow histiocytes and developmental plasmacytes. Proplasmacytes (pPC) in association with giant granulated histiocytes (ggh) and necrotic heterophils (HC). Additional descriptions are in the text.
Multinuclear forms
Multinuclear cells, a form of polyploidy, are common in MM. They may arise by incomplete mitosis (karyokinesis without cytokinesis) and by amitosis; examples are the subject of Figure 3. In panel A, a large (D 12. 8 μm) binuclear PC has oval nuclei of near equal size (5.6 μm; top; 5.4 μm, bottom) when measured on their long axes. Its deep blue cytoplasm displays a conspicuous Hof; small vacuoles are distributed throughout the cytoplasm. The chromatin of both nuclei is a “clock-face” configuration. The nuclear size equality suggests they are a product of incomplete mitosis. The small PC at the lower right of the binuclear PC has an N/C ~ 0.6, a diffuse Hof, and moderately basophilic cytoplasm. It could be classified as a “reactive” lymphocyte. In panel B, a small (D 7.9 μm) deeply basophilic binuclear PC displays a patchy Hof. The diameters of the nuclei measuring 3.3 μm (top) and 4.0 μm (bottom) indicate slight asymmetry, both display a “clock-face” chromatin configuration. Presumably this cell is also the product of incomplete mitosis. Mesomyelocytes (msm) and a faint HC are nearby.
A PC (cell 1) with a pair of large nuclei PC and basophilic cytoplasm is in panel C, its ovate shape suggests a recent incomplete mitosis. The central location of the Hof appears to service both nuclei. The cell immediately above (cell 2) is an example of a “hand-mirror” PC configuration with cytoplasmic thinning and elongation. These cells are seen in MM and acute leukemia9. The nearby HC show signs of necrosis.
A trinuclear PC with unequal nuclei is shown in panel D. The nuclear size heterogeneity suggests they are amitotic products often seen in blood of ducklings exposed to microbial toxins15. Although heterogeneous in size each nucleus displays a classic “clock-face” configuration. The cytoplasm, is noticeably less basophilic than PC or pPC of panels A, B, and C. The diffuse Hof is dispersed among the 3 nuclei. The earlier stages of this PC may have occurred at an extramedullary site, prior to migrating into the BM. The trinucleate cell is nested within a group of intact HC; a pPC with condensed chromatin is at the left.
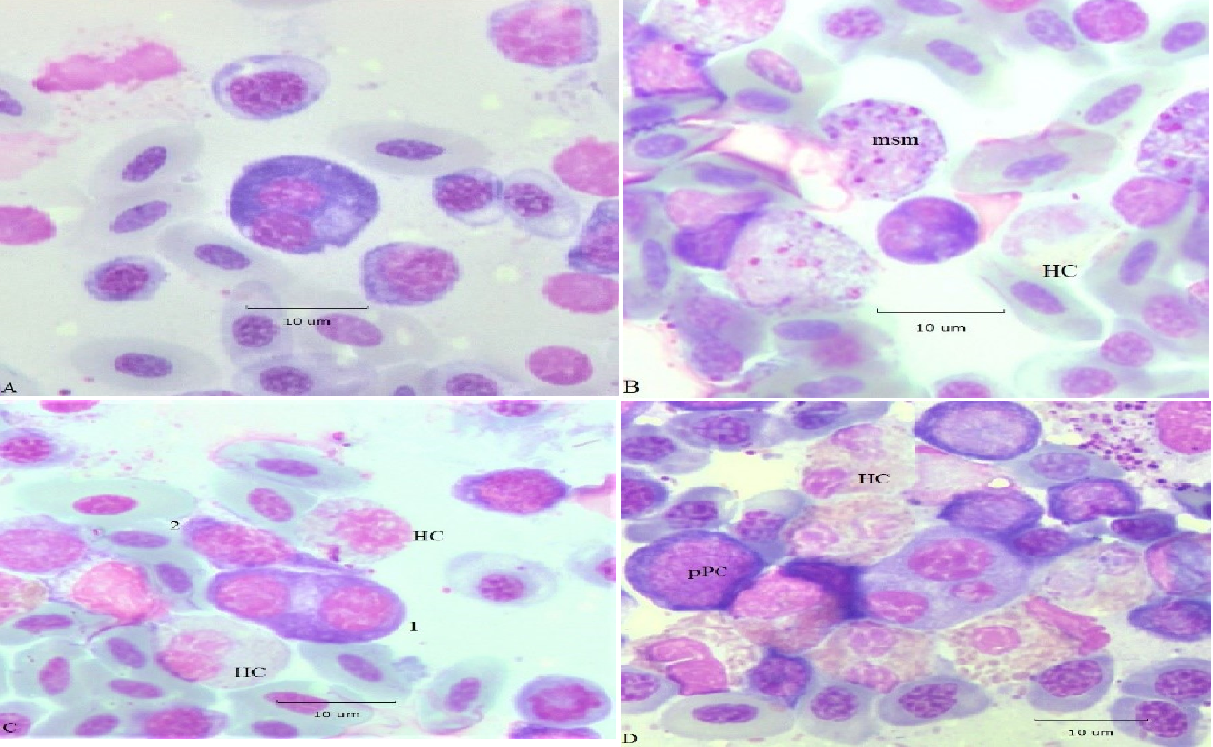
Figure 3: Atypical PC. Panel A, binuclear PC with a conspicuous Hof and small Russell bodies. Panel B small deeply basophilic binuclear PC with a patchy Hof. Panel C large binuclear PC with an equatorial Hof likely derived from incomplete mitosis; (2) “hand-mirror” PC. Panel D trinuclear PC with unequal sized nuclei; a proplasmacyte (pPC) and classic heterophils (HC). Additional descriptions are in the text.
Mott cells
Atypical plasmacytes with secretory defects, are known as Mott, grape, or morula cells. They are recognized by characteristic endoplasmic reticulum distensions (Russell bodies) resulting from accumulated Ig (reviewed in Hasegawa, 2013)16. Mott cells were in blood films of caged laying hens and in the bone marrow of lame broilers, and in ducks exposed to AFB15. More than 100 were detected in a single bone marrow smear from a 13-day lame duckling from which Streptococcus sp. were isolated. They were sorted on several criteria including cell diameter (D, long axis in μm) N/C ratio, size of RB (assigned: 0.25, 1.0, or 2.0 μm), and the presence of Dutcher bodies (nuclear RB equivalents). Size frequency and N/C distributions are in Figure 6.
A range of PC types appears in Figure 4 in association with a ggh. Cell 1, is a small (D 8.2 μm) “delta” shaped pPC with deep purple cytoplasm, a conspicuous Golgi, and has an N/C 0.5. Cell 2 is an early PC with a condensed nucleus and N/C 0.7. Cell 3 is a large Mott cell with heterogeneous sized RB (D 12.5 μm, N 6.9 μm; N/C 0.6) its eccentric nucleus contains Dutcher bodies. The largest RB are ~ 2.4 μm in diameter and the smallest are estimated as ~ 0.2 μm. The nucleus appears to be a “clock-face” type; however, its chromatin configuration is somewhat obscured because of Dutcher bodies. Cell 4 is a binuclear Mott with near symmetric nuclei (D 10.2, DN1 4.8 μm; DN2 5.0 μm). The cytoplasmic ranges from the deep hue of cell 1 to lighter shades of cells 2, 3, and 4.
Cells 5 and 6 are developmental stages of (HC) heterophils. The orange “specific” granules of cell 6 indicate it is nearly mature; cell 5 contains more primary (magenta) granules and so is earlier. Cell 7 is an eosinophil metamyelocyte. The large central ggh is surrounded by wreath of pRBC at a range of developmental stages. The micrometer occupies the place of a nuclear remnant likely from lysis of a giant (gh) histiocyte.
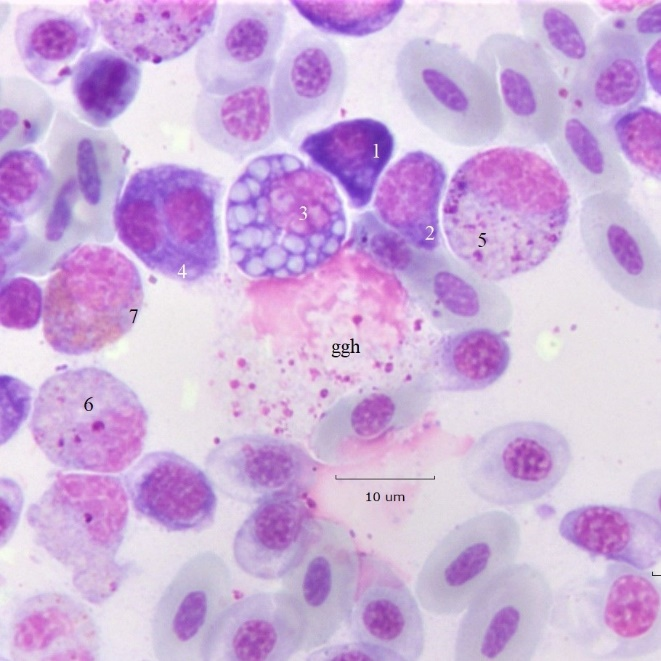
Figure 4: Mott cell variation. Examples of early proplasmacytes and Mott PC in the vicinity of a giant granular histiocyte and granulocyte meso/metamyelocytes. Additional descriptions are in the text.
Additional examples of Mott variants appear in Figure 5. Panel A. The large irregular shaped of cell 1 suggest it is a Mott type proplasmacyte likely at (arrested, multipolar) metaphase. Its Hof contains many small (< 1 μm) vacuoles; the cytoplasm is also fenestrated by extremely small vacuoles of a size approaching light microscopic resolution. Cell 2 is a small Dutcher type Mott cell (D ~ 5 μm) with heterogeneous RB, the largest are ~ 2 μm in diameter. Cell 3 is also a similarly sized Mott PC with small homogeneous RB (< 1μm). Cell 5 is a HC msm; cell 6 is a large eosinophilic msm. The remnants of a ggh occupy the center of the field. Background RBC are a mixture of mature (fully hemoglobinized) and immature (polychromatic) forms. Panel B. A ggh with a partially degraded RBC, a phagocytosed (? HC) apoptotic body (large arrow); bacteria are near the nucleus. Further away are intact encapsulated diplococci (presumably Streptococcus sp.) partially degraded bacilli and other smaller bacteria (small arrow). Panel C a central giant “orb” type Mott cell (area, A = 435.3 μm2; perimeter, P = 95.6 μm) with a partially lysed nucleus is surrounded by degraded HC and a pPC. Panel D. A giant orb type Mott cell (A = 202.5 μm2; P = 60.9 μm) with a partially lysed nucleus and a (ggh) are in the center of a mixed field populated by pPC and more developed PC. Cells of the heterophil series, meso (msm) and metamyelocytes (mtm) and necrotic heterophils (HC) are present. Early eosinophils (Eo) are at scattered locations. A binucleate PC with deep violet cytoplasm is at the lower left (bPC).
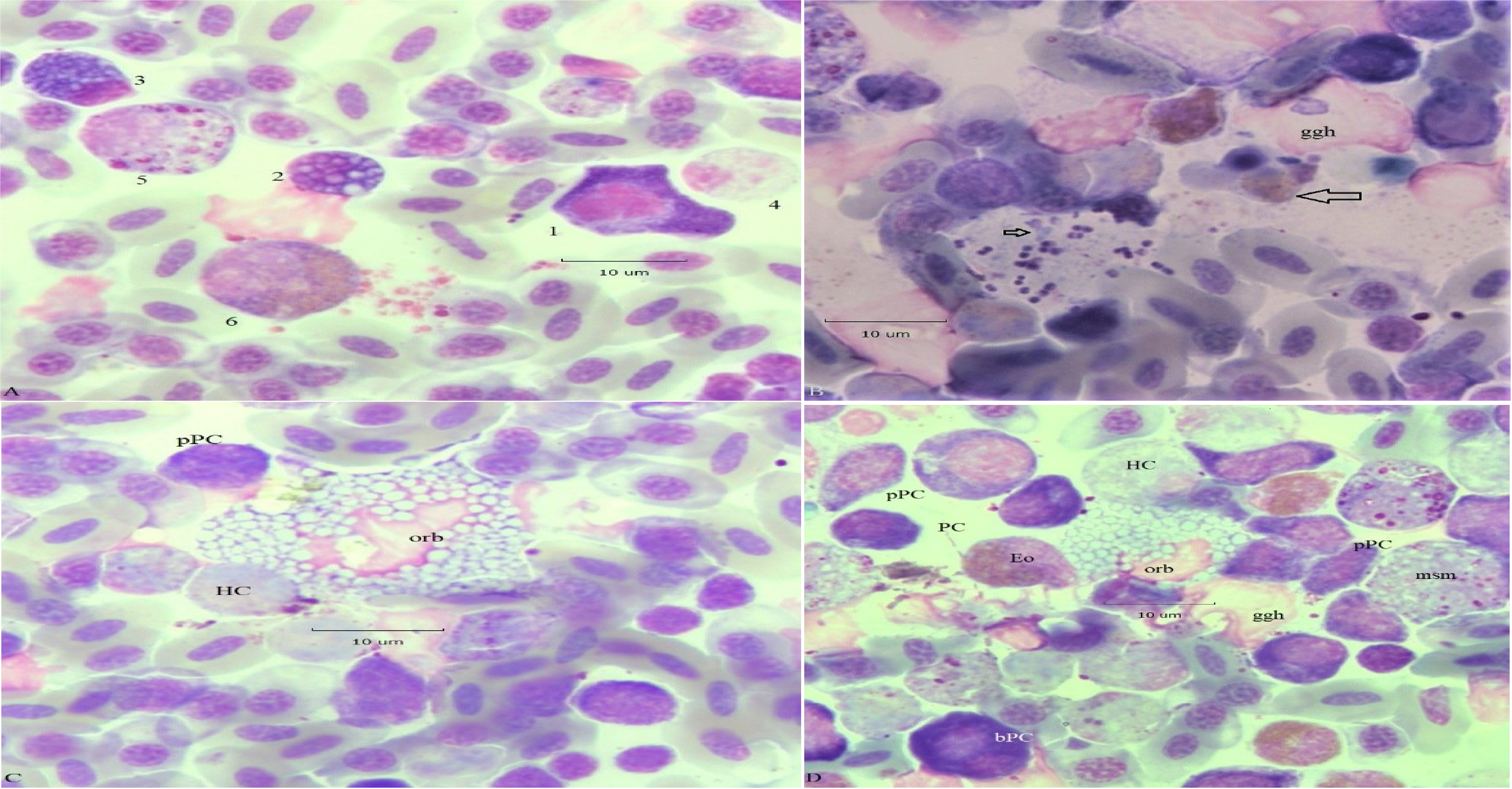
Figure 5: Mott variants. Panel A, Mott cell (1) in arrested mitosis. Panel B, bacteria of RP. Panel C, giant orb-type Mott cell (orb) with lysed nucleus. Panel D, orb-Mott cell (orb), binuclear proplasmacyte (bPC), and PC. Additional descriptions are in the text.
Although a few small cytoplasmic vacuoles may be found in a “normal” PC, a cell was declared as a Mott type if Dutcher and/or multiple Russell bodies were present. No attempt was made to further sub-categorize such cells on cytoplasmic hue criteria. Figure 6 (Left panel) gives their size frequency distribution from a single sample in which 102 Mott cells were observed. N/C ratios (Right panel) were measured in only 72/102 (71%) due to binucleate condition (N = 2), lysed nuclei (N = 21), mitotic figures (N = 2, Figure 5), and absence of nuclei (leukoplastids, N = 2).
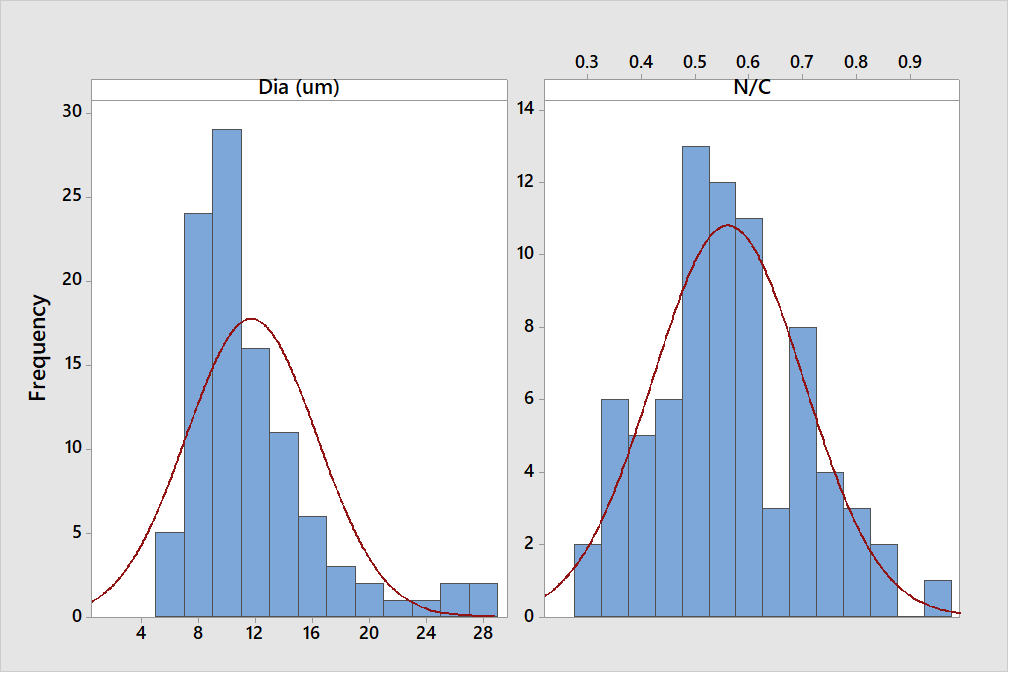
Figure 6: The characteristics of 72 of 102 Mott cells in the BM of a 13-d lame duckling. Left. The frequency distribution with normal curve of Mott-type PC diameters based on the longest axis of 102 cells. Right. N/C ratios with normal curve.
Discussion
Circulating PC, or an increase of bone marrow frequency, are cytological indications of active immunity. In normal chicken BM, PC are estimated at <1%2. In humans with reactive plasmacytosis PC increase to ~2% of BM8. While an exact frequency of PC in duckling BM was not computed in this study, PC frequencies were well above both the normal chicken value and the RP value for humans. This statement is supported by the numbers of pPC and PC among all cell types of figures 1-5.
It is presumed circulating PC are easily recognized microscopically because of distinctive morphology. While true in many cases, circulating PC were found among a continuum of reactive lymphocytes and other atypia in blood; making their differentiation from reactive lymphocytes more challenging3,4. More PC are in normal BM than in blood, but they occur as isolated cells and in scattered locations. In theory, after encountering antigen, B-cells divide into PC and memory cells. Some BM PC are thought to be long lived; others may not survive for more than a few days. However, both multinuclear PC, with the presumptive early (violet) hue, and the lighter blue shades occur in RP BM, indicating both types can survive long enough to complete one or more mitotic divisions.
It is not known if either the cytoplasmic hue or a multinuclear condition is associated with PC longevity. The cytoplasm spectrum topic has received little attention in the literature. The dark hues are similar to embryonic thymus lymphocytes described by Lucas2. Thus, it is presumed a dark PC cytoplasm represents a primitive condition, and the lighter cytoplasmic types represent a more derived state, consistent with what is the case in MM11. Interestingly a distinct Golgi is often seen in dark pPC/PC indicating that these presumptive early stages are able to secrete. Conversely, Mott cells whose cytoplasmic hues are also variable, and lack a capacity for secretion, can be mitotic and multinuclear.
The frequency distribution of Figure 6 indicates that the Mott condition (antibody retention in Russell bodies, RB) occurs in cells widely ranging in size. The N/C distribution of panel B shows the Mott condition occurs at multiple positions along the developmental pathway. Collectively, these observations challenge an idea long ago advanced that a Mott cell represents an end stage (senescence) in the life of a PC17. Moreover, some cells seen in MM contain RB; and as they are neoplastic, such cells are potentially immortal18.
Alternatively, because RB represent a form of cellular indigestion it is possible that certain Mott cell substances are actually injurious19. This might account for the lysed nuclei of “orb” type Mott cells described here (Figure 5). A novel form of nuclear lysis occurs in chicken heterophils20. Perhaps the lysed nuclei seen in orb-type Mott cells is a PC equivalent. To the author’s knowledge Figure 6 is the first such description of a Mott cell series.
It is not known why such a large number of Mott PC should appear in BM of lame ducklings. However, as a result of mutations, professional secretory cells, as are PC, can become toxic21. Moreover, truncated Ig variants lacking the first constant domain tend to condense into RB21. A portion of duck Ig antibody is known to exist in a truncated (5.7S) form lacking C3 and C4 domains, however22. Perhaps the high frequency of Mott cells in BM of lame ducklings is a consequence of protein folding difficulties associated with this peculiarity.
In summary, the present observations indicate that in RP, the PC series becomes highly heterogenous. Size, shape, nucleus number, a capacity for division, the condition (hue) of cytoplasm, the presence of accumulated antibody (RB) are sources of variation. Moreover, within BM, many PC associate with neighboring cells of both the histiocyte and granulocyte series; indicating variation of behavior. Association of 6 PC with a central standard sized human histiocyte in MM is known as “plasmacytic satellitosis”13. The nurturing cells here are giant size histiocytes however, and are either granulated or not (ggh/gh). To the author’s knowledge neither have been described elsewhere.
The advantage of studying PC variation in reactive circumstances, is that it provides an opportunity to compare non-neoplastic reactive types with neoplastic types as occur in diseases as MM and leukemia. This supports the idea of a continuity between reactive and neoplastic conditions. Studies such as this will be greatly aided by development of stage specific (IHC) reagents with the capacity to further differentiate the PC varieties described here. In the future, it may be possible to associate a particular reactive PC variant with a specific clinical condition.
Acknowledgements
The author is grateful to Mr. Ben Fetrow of Maple Leaf Farms, Leesburg, IN 46538 who selected the ducklings and made the slides.
Abbreviations used in the text
A, area μm2, BM, bone marrow, D, cell diameter, N, nuclear diameter, gh, giant histiocyte, ggh, giant granulated histiocyte, HC, classic heterophil, HT, typical heterophil, MM, multiple myeloma, N/C, nuclear/cytoplasmic (area) ratio, P, perimeter μm, PC, plasmacyte, bPC, binuclear plasmacyte, pPC, proplasmacyte, pRBC, polychromatic erythrocyte, RP, reactive plasmacytosis.
References
- Masteller EL, GT Pharr, PE Funk, et al, 1997. Avian B cell development. Int. Rev. Immunol.15(3-4):185-206. doi: 10.3109/08830189709068176. PMID: 9222819
- Lucas AM, and C Jamroz. 1961. Atlas of Avian Hematology. Monograph 25. Washington: U.S. Dept. of Agriculture.
- Cotter PF, 2015a. Atypical lymphocytes and leukocytes in the peripheral circulation of caged hens. Poult. Sci. 94: 1439–1445. http://dx.doi.org/10.3382/ps/pev157.
- Cotter PF, 2015b. Are peripheral Mott cells an indication of stress or inefficient immunity? Poult. Sci. 2015 Jul; 94(7): 1433-8. doi: 10.3382/ps/pev053. Epub 2015 Feb 27. PMID: 25725075.
- Cotter PF and M. Bakst, 2017. A comparison of Mott cell morphology of three avian species. II. – Bad behavior by plasmocytes? Poult. Sci. 96: 325-331 http://dx.doi.org/10.3382/ps/pew288.
- Cotter PF and ED. Heller, 2016. Complex hemograms of isolator raised specific pathogen free (SPF) chicks. International Journal of Poultry Science, 15: 211-217.DOI: 10.3923/ijps.2016.211.217URL: https://scialert.net/abstract/?doi=ijps.2016.211.217
- Corre JN, Munshi H. Avet-Loiseau, 2015. Genetics of multiple myeloma: another heterogeneity level? Blood 125(12): 1870-1876. doi:10.1182/blood-2014-10-567370
- Hyun BH, D. Kwa, et al, 1976. Reactive plasmacytic lesions of the bone marrow. Am. J. Clin. Pathol. 65: 921-928. doi: 10.1093/ajcp/65.6.921
- Greipp PR, NM. Raymond, RA. Kyle, et al., 1985. Multiple Myeloma: significance of plasmablastic subtype in morphological classification. Blood 65(2): 305-310 ISSN 0006-4971, https://doi.org/10.1182/blood.
- Campbell TW and CK. Ellis, 2007. Page 19, Avian and Exotic Animal Hematology and Cytology, 3rd ed. Blackwell Pub., Ames, IA.
- Ribourtout B, M. Zandecki, 2015. Plasma cell morphology in multiple myeloma and related disorders. Morphologie 99 (325): 38-62 ISSN12860115.https://doi.org/10.1016/j.morpho.2015.02.001
- Cotter PF, 2021. Erythroplastids of duck blood produced by cytokinesis, lysis, and amitosis. J. World Poult. Res. 11(2): 271-277 DOI: https://dx.doi.org/10.36380/jwpr.2021.32.
- Pillay TS, G. Sayers, et al., 1992. Plasmacyte-reticulum cell satellitism in multiple myeloma associated with amyloidosis. J. Clin. Pathol. 45(7): 623-4. doi: 10.1136/jcp.45.7.623. PMID: 1517465; PMCID: PMC495193.
- Minges Wols HA, 2015. Plasma Cells. Wiley Online Library https://doi.org/10.1002/9780470015902.a0004030.pub2.
- Cotter PF, 2021. Atypical hemograms of the commercial duck. Poult Sci.100(8):101248. doi: 10.1016/j.psj.2021.101248. Epub 2021 May 13. PMID: 34225201; PMCID: PMC8264152.
- Hasegawa H. 2013, Review Article: Aggregates, crystals, gels, and amyloids: intracellular and extracellular phenotypes at the crossroads of immunoglobulin physicochemical property and cell physiology. Int. J. Cell Biol. 2013, Article ID 604867, http://dx.doi.org/10.1155/2013/604867.
- Miller FR, 1931. The induced development and histogenesis of plasma cells. J. Exp. Med. 54(3): 333-347. doi: 10.1084/jem.54.3.333. PMID: 19869921; PMCID: PMC2132006.
- van Gammeren AJ, LS. Alcala, et al., 2015. Numerous Russell bodies and Dutcher bodies in multiple myeloma. Br. J. Haematol. 170(6): 743. doi: 10.1111/bjh.13181. Epub 2014 Oct 14. PMID: 25312850.
- Kopito RR, R. Sitia, 2000. Aggresomes and Russell bodies. Symptoms of cellular indigestion? EMBO Rep. 1(3): 225-231. doi:10.1093/embo-reports/kvd052
- Cotter PF., 2021. Apoptosis of circulating heterophils; implications for the interpretation of the heterophil/lymphocyte ratio. J. Immunological Sci. 5(4): 1-8.doi: 10.29245/2578-3009/2021/4.1222
- Mossuto F, D. Ami, et al., 2015. Biochemical nature of Russell Bodies. Sci. Rep. 5, 12585; doi: 10.1038/srep12585
- Magor KE, DA. Higgins, et al., 1994. One gene encodes the heavy chains for three different forms of IgY in the duck. J. Immunol. 15; 153(12): 5549-55. PMID: 7989756
